Evaluation of Wearable Digital Devices in a Phase I Clinical Trial
- PMID: 30635980
- PMCID: PMC6510458
- DOI: 10.1111/cts.12602
Evaluation of Wearable Digital Devices in a Phase I Clinical Trial
Abstract
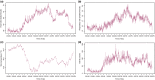
We assessed the performance of two US Food and Drug Administration (FDA) 510(k)-cleared wearable digital devices and the operational feasibility of deploying them to augment data collection in a 10-day residential phase I clinical trial. The Phillips Actiwatch Spectrum Pro (Actiwatch) was used to assess mobility and sleep, and the Vitalconnect HealthPatch MD (HealthPatch) was used for monitoring heart rate (HR), respiratory rate (RR), and surface skin temperature (ST). We measured data collection rates, compared device readouts with anticipated readings and conventional in-clinic measures, investigated data limitations, and assessed user acceptability. Six of nine study participants consented; completeness of data collection was adequate (> 90% for four of six subjects). A good correlation was observed between the HealthPatch device derived and in-clinic measures for HR (Pearson r = 0.71; P = 2.2e-16) but this was poor for RR (r = 0.08; P = 0.44) and ST (r = 0.14; P = 0.14). Manual review of electrocardiogram strips recorded during reported episodes of tachycardia > 180 beats/min showed that these were artefacts. The HealthPatch was judged to be not fit-for-purpose because of artefacts and the need for time-consuming manual review. The Actiwatch device was suitable for monitoring mobility, collecting derived sleep data, and facilitating the interpretation of vital sign data. These results suggest the need for fit-for-purpose evaluation of wearable devices prior to their deployment in drug development studies.
© 2018 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
E.S.I., I.L.M., G.H., D.M., E.D.P., and J.A.W. are employees of Takeda Pharmaceuticals and own stock in the company. G.B., M.C., and C.B. are employees of Koneksa Health and own stock in the company. As Editor‐in‐Chief for
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

