Imaging Bioluminescent Exogenous Stem Cells in the Intact Guinea Pig Cochlea
- PMID: 30635981
- PMCID: PMC7065152
- DOI: 10.1002/ar.24068
Imaging Bioluminescent Exogenous Stem Cells in the Intact Guinea Pig Cochlea
Abstract
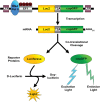
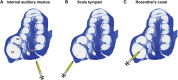
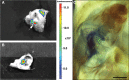
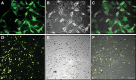
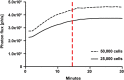
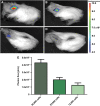
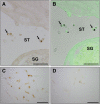
Stem-cell-based therapy may be used to replace damaged or lost neurons in the cochlear nerve of patients suffering from severe-to-profound sensorineural hearing loss. In order to achieve functional recovery in future clinical trials, knowledge about survival of grafted cells and their differentiation into functional neurons is a prerequisite. This calls for non-invasive in vivo visualization of cells and long-term monitoring of their survival and fate after cochlear transplantation. We have investigated if molecular optical imaging enables visualization of exogenous cells in the intact cochlea of guinea pig cadaver heads. Transduced (stem) cells, stably co-expressing fluorescent (copGFP) and bioluminescent (Luc2) reporter molecules, were injected into the internal auditory meatus or directly into the cochlea through the round window. After injection of the cells into the internal auditory meatus, a bright bioluminescent signal was observed in the cavum conchae of the auricle, indicating that light generated by Luc2 is passing through the tympanic membrane and the external auditory meatus. Similar results were obtained after injection of the cells through the round window membrane, either directly into the scala tympani or in Rosenthal's canal within the modiolus of the basal cochlear turn. Imaging of the auditory bulla demonstrated that the bioluminescent signal passes through the tympanic membrane and crevices in the bony wall of the bulla. After opening the auditory bulla, the bioluminescent signal was emanating from the round window. This is the first study demonstrating that bioluminescence imaging enables visualization of luciferase-expressing cells injected into the intact guinea pig cochlea. Anat Rec, 303:427-440, 2020. © 2019 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association of Anatomists.
Keywords: bioluminescence imaging; cochlea; firefly luciferase; guinea pig; hair-follicle-bulge-derived stem cells.
© 2019 The Authors. The Anatomical Record published by Wiley Periodicals, Inc. on behalf of American Association of Anatomists.
Figures

References
-
- Bogaerts S, Douglas S, Corlette T, Pau H, Saunders D, McKay S, Oleskevich S. 2008. Microsurgical access for cell injection into the mammalian cochlea. J Neurosci Methods 168:156–163. - PubMed
-
- Briaire JJ, Frijns JHM. 2006. The consequences of neural degeneration regarding optimal cochlear implant position in scala tympani: A model approach. Hear Res 214:17–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

