Pretreatment With PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation
- PMID: 30636486
- PMCID: PMC6497363
- DOI: 10.1161/JAHA.118.010838
Pretreatment With PCSK9 Inhibitor Protects the Brain Against Cardiac Ischemia/Reperfusion Injury Through a Reduction of Neuronal Inflammation and Amyloid Beta Aggregation
Abstract
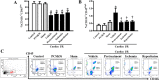
Background Cardiac ischemic/reperfusion (I/R) injury leads to brain damage. A new antihyperlipidemic drug is aimed at inhibiting PCSK 9 (proprotein convertase subtilisin/kexin type 9), a molecule first identified in a neuronal apoptosis paradigm. Thus, the PCSK 9 inhibitor ( PCSK 9i) may play a role in neuronal recovery following cardiac I/R insults. We hypothesize that PCSK 9i attenuates brain damage caused by cardiac I/R via diminishing microglial/astrocytic hyperactivation, β-amyloid aggregation, and loss of dendritic spine. Methods and Results Adult male rats were divided into 7 groups: (1) control (n=4); (2) PCSK 9i without cardiac I/R (n=4); (3) sham (n=4); and cardiac I/R (n=40). Cardiac I/R rats were divided into 4 subgroups (n=10/subgroup): (1) vehicle; (2) PCSK 9i (10 μg/kg, IV) before ischemia; (3) PCSK 9i during ischemia; and (4) PCSK 9i at the onset of reperfusion. At the end of cardiac I/R protocol, brains were removed to determine microglial and astrocytic activities, β-amyloid aggravation, and dendritic spine density. The cardiac I/R led to the activation of the brain's innate immunity resulting in increasing Iba1+ microglia, GFAP + astrocytes, and CD 11b+/ CD 45+high cell numbers. However, CD 11b+/ CD 45+low cell numbers were decreased following cardiac I/R. In addition, cardiac I/R led to reduced dendritic spine density, and increased β-amyloid aggregation. Only the administration of PCSK 9i before ischemia effectively attenuated these deleterious effects on the brain following cardiac I/R. PCSK 9i administration under the physiologic condition did not affect the aforementioned parameters. Conclusions Cardiac I/R injury activated microglial activity in the brain, leading to brain damage. Only the pretreatment with PCSK 9i prevented dendritic spine loss via reduction of microglial activation and Aβ aggregation.
Keywords: amyloid; brain; ischemia/reperfusion injury/neuroprotection; proprotein convertase subtilisin/kexin type 9.
Figures





References
-
- Palmerini T, Tomasi L, Barozzi C, Della Riva D, Mariani A, Taglieri N, Leone O, Ceccarelli C, De Servi S, Branzi A, Genereux P, Stone GW, Ahamed J. Detection of tissue factor antigen and coagulation activity in coronary artery thrombi isolated from patients with ST‐segment elevation acute myocardial infarction. PLoS One. 2013;8:e81501. - PMC - PubMed
-
- Ramirez‐Sanchez J, Pires ENS, Meneghetti A, Hansel G, Nunez‐Figueredo Y, Pardo‐Andreu GL, Ochoa‐Rodríguez E, Verdecia‐Reyes Y, Delgado‐Hernández R, Salbego C, Souza DO. JM‐20 treatment after MCAO reduced astrocyte reactivity and neuronal death on peri‐infarct regions of the rat brain. Mol Neurobiol. 2018. Available at: https://link.springer.com/article/10.1007%2Fs12035-018-1087-8. Accessed January 3, 2019. - PubMed
-
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70:1082. - PubMed
-
- Kumfu S, Charununtakorn ST, Jaiwongkam T, Chattipakorn N, Chattipakorn SC. Humanin prevents brain mitochondrial dysfunction in a cardiac ischaemia‐reperfusion injury model. Exp Physiol. 2016;101:697–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

