Oxidative and Inflammatory Events in Prion Diseases: Can They Be Therapeutic Targets?
- PMID: 30636622
- PMCID: PMC6635421
- DOI: 10.2174/1874609812666190111100205
Oxidative and Inflammatory Events in Prion Diseases: Can They Be Therapeutic Targets?
Abstract
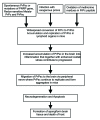
Prion diseases are a group of incurable infectious terminal neurodegenerative diseases caused by the aggregated misfolded PrPsc in selected mammals including humans. The complex physical interaction between normal prion protein PrPc and infectious PrPsc causes conformational change from the α- helix structure of PrPc to the β-sheet structure of PrPsc, and this process is repeated. Increased oxidative stress is one of the factors that facilitate the conversion of PrPc to PrPsc. This overview presents evidence to show that increased oxidative stress and inflammation are involved in the progression of this disease. Evidence is given for the participation of redoxsensitive metals Cu and Fe with PrPsc inducing oxidative stress by disturbing the homeostasis of these metals. The fact that some antioxidants block the toxicity of misfolded PrPc peptide supports the role of oxidative stress in prion disease. After exogenous infection in mice, PrPsc enters the follicular dendritic cells where PrPsc replicates before neuroinvasion where they continue to replicate and cause inflammation leading to neurodegeneration. Therefore, reducing levels of oxidative stress and inflammation may decrease the rate of the progression of this disease. It may be an important order to reduce oxidative stress and inflammation at the same time. This may be achieved by increasing the levels of antioxidant enzymes by activating the Nrf2 pathway together with simultaneous administration of dietary and endogenous antioxidants. It is proposed that a mixture of micronutrients could enable these concurrent events thereby reducing the progression of human prion disease.
Keywords: Oxidative stress; antioxidants; apoptosis; inflammation; misfolded proteins; prion diseases; spongiform encephalopathy..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
-
- Gajdusek D.C., Zigas V. Degenerative disease of the central nervous system in New Guinea; the endemic occurrence of kuru in the native population. N. Engl. J. Med. 1957;257(20):974–978. - PubMed
-
- Gibbs C.J., Gajdusek D.C., Asher D.M., et al. Creutzfeldt-Jakob disease (spongiform encephalopathy): Transmission to the chimpanzee. Science. 1968;161(3839):388–389. - PubMed
-
- Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. - PubMed
-
- Chen Y.R., Yi F.F., Li X.Y., et al. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc. Drugs Ther. 2008;22(6):479–485. - PubMed