CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice
- PMID: 30636629
- PMCID: PMC6330571
- DOI: 10.1186/s13195-018-0461-0
CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice
Abstract
Background: The annual incidence of traumatic brain injury (TBI) in the United States is over 2.5 million, with approximately 3-5 million people living with chronic sequelae. Compared with moderate-severe TBI, the long-term effects of mild TBI (mTBI) are less understood but important to address, particularly for contact sport athletes and military personnel who have high mTBI exposure. The purpose of this study was to determine the behavioural and neuropathological phenotypes induced by the Closed-Head Impact Model of Engineered Rotational Acceleration (CHIMERA) model of mTBI in both wild-type (WT) and APP/PS1 mice up to 8 months post-injury.
Methods: Male WT and APP/PS1 littermates were randomized to sham or repetitive mild TBI (rmTBI; 2 × 0.5 J impacts 24 h apart) groups at 5.7 months of age. Animals were assessed up to 8 months post-injury for acute neurological deficits using the loss of righting reflex (LRR) and Neurological Severity Score (NSS) tasks, and chronic behavioural changes using the passive avoidance (PA), Barnes maze (BM), elevated plus maze (EPM) and rotarod (RR) tasks. Neuropathological assessments included white matter damage; grey matter inflammation; and measures of Aβ levels, deposition, and aducanumab binding activity.
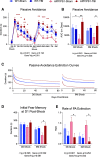
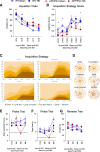
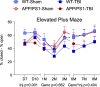
Results: The very mild CHIMERA rmTBI conditions used here produced no significant acute neurological or motor deficits in WT and APP/PS1 mice, but they profoundly inhibited extinction of fear memory specifically in APP/PS1 mice over the 8-month assessment period. Spatial learning and memory were affected by both injury and genotype. Anxiety and risk-taking behaviour were affected by injury but not genotype. CHIMERA rmTBI induced chronic white matter microgliosis, axonal injury and astrogliosis independent of genotype in the optic tract but not the corpus callosum, and it altered microgliosis in APP/PS1 amygdala and hippocampus. Finally, rmTBI did not alter long-term tau, Aβ or amyloid levels, but it increased aducanumab binding activity.
Conclusions: CHIMERA is a useful model to investigate the chronic consequences of rmTBI, including behavioural abnormalities consistent with features of post-traumatic stress disorder and inflammation of both white and grey matter. The presence of human Aβ greatly modified extinction of fear memory after rmTBI.
Keywords: Alzheimer disease mice; Aβ metabolism; CHIMERA; Neuroinflammation; Post-traumatic stress disorder; Spatial memory; Traumatic brain injury.
Conflict of interest statement
Ethics approval and consent to participate
All experiments were approved by the University of British Columbia Committee on Animal Care and are compliant with Canadian Council of Animal Care (A15-0096) guidelines.
Consent for publication
All authors have consented to publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, Sanberg PR, Bickford PC, Kaneko Y, Borlongan CV. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One. 2013;8(1):e53376. doi: 10.1371/journal.pone.0053376. - DOI - PMC - PubMed
-
- Bajwa NM, Halavi S, Hamer M, Semple BD, Noble-Haeusslein LJ, Baghchechi M, Hiroto A, Hartman RE, Obenaus A. Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PLoS One. 2016;11(1):e0146886. doi: 10.1371/journal.pone.0146886. - DOI - PMC - PubMed
-
- Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, Greig NH, Pick CG. Tumor necrosis factor-α synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem. 2011;118(6):1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

