Minimally invasive spinal anesthesia for cesarean section in maternal anticoagulation therapy: a randomized controlled trial
- PMID: 30636632
- PMCID: PMC6330402
- DOI: 10.1186/s12871-018-0679-1
Minimally invasive spinal anesthesia for cesarean section in maternal anticoagulation therapy: a randomized controlled trial
Abstract
Background: Anticoagulant therapy during pregnancy is widely used due to the increasing awareness of maternal hypercoagulability. Few studies have reported the use of minimally invasive spinal anesthesia in these parturients. The objective of this study was to evaluate the safety and feasibility of minimally invasive spinal anesthesia in parturients with anticoagulation therapy undergoing cesarean section.
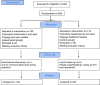
Methods: This was a randomized, controlled study conducted in 239 parturients using anticoagulants and undergoing selective cesarean section. 37 parturients withdrew, and finally parturients received spinal anesthesia using 27gauge pen type fine spinal needles (experimental group, n = 110) and 22gauge traditional spinal needles (control group, n = 92). The primary efficacy outcomes included low back pain (LBP) and postdural puncture headache (PDPH) after delivery. Secondary efficacy outcomes included visual analogue scale during subarachnoid puncture (VASdural), difference between visual analogue scale (VAS) during peripheral venipuncture and VASdural (∆VAS), VAS of back puncture point 24, 48 and 72 h after operation (VASdural-24 h, VASdural-48 h and VASdural-72 h, respectively), maternal satisfaction and hospitalization stay.
Results: No parturient had PDPH and was suspected with spinal or intracranial haematoma in two groups. There was no significant difference in VASlbp-24 h, VASlbp-48 h and VASlbp-72 h (P = 0.056; P = 0.813; P = 0.189, respectively) between two groups. In experimental group, VASdural (P = 0.017), ∆VAS (P = 0.001) and VASdural-24 h (P < 0.0001) were lower, whereas maternal satisfaction was higher (P = 0.046). There was no significant difference in VASdural-48 h, VASdural-72 h, urination function, strength recovery and hospitalization stay (P = 0.069; P = 0.667; P = 0.105; P = 0.133; P = 0.754, respectively) between the two groups.
Conclusions: Minimally invasive spinal anesthesia provided lower VASdural, VASdrual-24 h and a higher maternal satisfaction. Hence, it is considered as a safe, reliable and reasonable option for cesarean section parturients during maternal anticoagulation therapy with normal platelet count and coagulation time.
Trial registration: This study was registered at www.ClinicalTrials.gov at November 11th, 2016 ( NCT02987192 ).
Keywords: Anticoagulants; Cesarean section; Low back pain; Minimally invasive; Postdural puncture headache; Spinal anesthesia.
Conflict of interest statement
Ethics approval and consent to participate
The Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiaotong University reviewed the study protocol and the ethical approval was granted in March 2016 (No. 2016–033). Written informed consents were obtained either from the patients or legal representatives.
Consent for publication
All included patients or their family members signed the informed consent form to report individual patient data. All authors have confirmed the manuscript and approved the publication of the manuscript.
Competing interests
No conflict of interest was declared by any of the authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Impact of spinal needle type on postdural puncture headache among women undergoing Cesarean section surgery under spinal anesthesia: A meta-analysis.J Evid Based Med. 2018 Aug;11(3):136-144. doi: 10.1111/jebm.12311. Epub 2018 Aug 1. J Evid Based Med. 2018. PMID: 30070060
-
Effect of ondansetron on post-dural puncture headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study.J Anesth. 2015 Oct;29(5):702-7. doi: 10.1007/s00540-015-2000-5. Epub 2015 Mar 27. J Anesth. 2015. PMID: 25812804 Clinical Trial.
-
Impact of spinal needle design and approach to postdural puncture headache and spinal anesthesia failure in obstetrics.Anaesthesiol Intensive Ther. 2019;51(2):77-82. doi: 10.5114/ait.2019.86166. Anaesthesiol Intensive Ther. 2019. PMID: 31268266 Clinical Trial.
-
Efficacy and potency of sphenopalatine ganglion block for the management of postdural puncture headaches in post-cesarean section: A case report and literature review.J Obstet Gynaecol Res. 2024 Dec;50(12):2357-2361. doi: 10.1111/jog.16121. Epub 2024 Oct 13. J Obstet Gynaecol Res. 2024. PMID: 39400462 Free PMC article. Review.
-
Anesthetic Considerations in the Obese Parturient.Clin Obstet Gynecol. 2016 Mar;59(1):193-203. doi: 10.1097/GRF.0000000000000180. Clin Obstet Gynecol. 2016. PMID: 26694495 Review.
Cited by
-
Popliteal sciatic nerve block versus intrathecal anesthesia for Achilles tendon rupture repair surgery: a mono-centric retrospective comparative study.Front Med (Lausanne). 2025 Jul 21;12:1516874. doi: 10.3389/fmed.2025.1516874. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40761867 Free PMC article.
-
Low-dose intranasal dexmedetomidine premedication improves epidural labor analgesia onset and reduces procedural pain on epidural puncture: a prospective randomized double-blind clinical study.BMC Anesthesiol. 2023 May 30;23(1):185. doi: 10.1186/s12871-023-02146-5. BMC Anesthesiol. 2023. PMID: 37254106 Free PMC article. Clinical Trial.
References
-
- Lumbiganon P, Laopaiboon M, Gulmezoglu AM, Souza JP, Taneepanichskul S, Ruyan P, Attygalle DE, Shrestha N, Mori R, Nguyen DH, et al. Method of delivery and pregnancy outcomes in Asia: the WHO global survey on maternal and perinatal health 2007-08. Lancet. 2010;375(9713):490–499. doi: 10.1016/S0140-6736(09)61870-5. - DOI - PubMed
-
- Appropriate technology for birth. Lancet. 1985;2(8452):436–7. 10.1016/S0140-6736(85)92750-3. - PubMed
-
- Kuczkowski KM. Post-dural puncture headache in the obstetric patient: an old problem. New solutions. Minerva Anestesiol. 2004;70(12):823–830. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous