Oxidative stress induces club cell proliferation and pulmonary fibrosis in Atp8b1 mutant mice
- PMID: 30636723
- PMCID: PMC6339797
- DOI: 10.18632/aging.101742
Oxidative stress induces club cell proliferation and pulmonary fibrosis in Atp8b1 mutant mice
Abstract
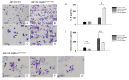
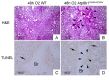
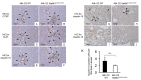
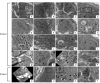
Atp8b1 (ATPase, aminophospholipid transporter, class I, type 8B, member 1) is a cardiolipin transporter in the apical membrane of lung epithelial cells. While the role of Atp8b1 in pneumonia-induced acute lung injury (ALI) has been well studied, its potential role in oxidative stress-induced ALI is poorly understood. We herein show that Atp8b1G308V/G308V mice under hyperoxic conditions display exacerbated cell apoptosis at alveolar epithelium and aberrant proliferation of club cells at bronchiolar epithelium. This hyperoxia-induced ambivalent response in Atp8b1G308V/G308V lungs was followed by patchy distribution of non-uniform interstitial fibrosis at late recovery phase under normoxia. Since this club cell abnormality is commonly observed between Atp8b1G308V/G308V lungs under hyperoxic conditions and IPF lungs, we characterized this mouse fibrosis model focusing on club cells. Intriguingly, subcellular morphological analysis of IPF lungs, using transmission electron microscopy (TEM), revealed that metaplastic bronchiolar epithelial cells in fibrotic lesions and deformed type II alveolar epithelial cells (AECs) in alveoli with mild fibrosis, have common morphological features including cytoplasmic vacuolation and dysmorphic lamellar bodies. In conclusion, the combination of Atp8b1 mutation and hyperoxic insult serves as a novel platform to study unfocused role of club cells in IPF.
Keywords: Atp8b1; club cells; hyperoxia; idiopathic pulmonary fibrosis (IPF); oxidative stress.
Conflict of interest statement
Figures








Similar articles
-
Matrix Metalloproteinase 7 Expression and Apical Epithelial Defects in Atp8b1 Mutant Mouse Model of Pulmonary Fibrosis.Biomolecules. 2022 Feb 9;12(2):283. doi: 10.3390/biom12020283. Biomolecules. 2022. PMID: 35204783 Free PMC article.
-
Global gene profiling of aging lungs in Atp8b1 mutant mice.Aging (Albany NY). 2016 Sep 29;8(9):2232-2252. doi: 10.18632/aging.101056. Aging (Albany NY). 2016. PMID: 27689529 Free PMC article.
-
The phospholipid flippase ATP8B1 mediates apical localization of the cystic fibrosis transmembrane regulator.Biochim Biophys Acta. 2016 Sep;1863(9):2280-8. doi: 10.1016/j.bbamcr.2016.06.005. Epub 2016 Jun 11. Biochim Biophys Acta. 2016. PMID: 27301931
-
Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers.Proc Am Thorac Soc. 2006 Jun;3(4):364-72. doi: 10.1513/pats.200601-003TK. Proc Am Thorac Soc. 2006. PMID: 16738202 Review.
-
[Pathogenesis of idiopathic interstitial pneumonia/idiopathic pulmonary fibrosis: cellular and molecular biology of the disease].Nihon Kyobu Shikkan Gakkai Zasshi. 1993 Dec;31 Suppl:20-31. Nihon Kyobu Shikkan Gakkai Zasshi. 1993. PMID: 8007466 Review. Japanese.
Cited by
-
Clinical manifestations and treatment outcomes among hospitalised COVID-19 patients in tertiary hospitals in Tanzania, 2021-2022: a retrospective cohort study.BMJ Public Health. 2024 Aug 16;2(2):e000881. doi: 10.1136/bmjph-2023-000881. eCollection 2024 Dec. BMJ Public Health. 2024. PMID: 40018602 Free PMC article.
-
Oxygen: Friend or foe in the COVID-19 battle.Clin Case Rep. 2021 Dec 26;9(12):e05254. doi: 10.1002/ccr3.5254. eCollection 2021 Dec. Clin Case Rep. 2021. PMID: 34963816 Free PMC article.
-
Matrix Metalloproteinase 7 Expression and Apical Epithelial Defects in Atp8b1 Mutant Mouse Model of Pulmonary Fibrosis.Biomolecules. 2022 Feb 9;12(2):283. doi: 10.3390/biom12020283. Biomolecules. 2022. PMID: 35204783 Free PMC article.
-
ATP8B1 Knockdown Activated the Choline Metabolism Pathway and Induced High-Level Intracellular REDOX Homeostasis in Lung Squamous Cell Carcinoma.Cancers (Basel). 2022 Feb 7;14(3):835. doi: 10.3390/cancers14030835. Cancers (Basel). 2022. PMID: 35159102 Free PMC article.
-
A-Kinase Anchor Protein 1 deficiency causes mitochondrial dysfunction in mouse model of hyperoxia induced acute lung injury.Front Pharmacol. 2022 Oct 3;13:980723. doi: 10.3389/fphar.2022.980723. eCollection 2022. Front Pharmacol. 2022. PMID: 36263130 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

