Overproduction of native endo-β-1,4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice
- PMID: 30636971
- PMCID: PMC6325865
- DOI: 10.1186/s13068-018-1351-1
Overproduction of native endo-β-1,4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice
Abstract
Background: Genetic modification of plant cell walls has been implemented to reduce lignocellulosic recalcitrance for biofuel production. Plant glycoside hydrolase family 9 (GH9) comprises endo-β-1,4-glucanase in plants. Few studies have examined the roles of GH9 in cell wall modification. In this study, we independently overexpressed two genes from GH9B subclasses (OsGH9B1 and OsGH9B3) and examined cell wall features and biomass saccharification in transgenic rice plants.
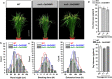
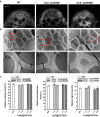
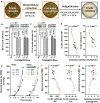
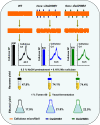
Results: Compared with the wild type (WT, Nipponbare), the OsGH9B1 and OsGH9B3 transgenic rice plants, respectively, contained much higher OsGH9B1 and OsGH9B3 protein levels and both proteins were observed in situ with nonspecific distribution in the plant cells. The transgenic lines exhibited significantly increased cellulase activity in vitro than the WT. The OsGH9B1 and OsGH9B3 transgenic plants showed a slight alteration in three wall polymer compositions (cellulose, hemicelluloses, and lignin), in their stem mechanical strength and biomass yield, but were significantly decreased in the cellulose degree of polymerization (DP) and lignocellulose crystalline index (CrI) by 21-22%. Notably, the crude cellulose substrates of the transgenic lines were more efficiently digested by cellobiohydrolase (CBHI) than those of the WT, indicating the significantly increased amounts of reducing ends of β-1,4-glucans in cellulose microfibrils. Finally, the engineered lines generated high sugar yields after mild alkali pretreatments and subsequent enzymatic hydrolysis, resulting in the high bioethanol yields obtained at 22.5% of dry matter.
Conclusions: Overproduction of OsGH9B1/B3 enzymes should have specific activity in the postmodification of cellulose microfibrils. The increased reducing ends of β-1,4-glucan chains for reduced cellulose DP and CrI positively affected biomass enzymatic saccharification. Our results demonstrate a potential strategy for genetic modification of cellulose microfibrils in bioenergy crops.
Keywords: Bioethanol production; Biomass saccharification; Cellulose modification; Chemical pretreatment; Endo-β-1,4-glucanases; GH9B; Transgenic rice.
Figures





References
-
- Domínguez-Escribà L, Porcar M. Rice straw management: the big waste. Biofuels Bioprod Bioref. 2009;4:154–159. doi: 10.1002/bbb.196. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

