Large Disparity between Prevalence and Treatment Rates for Hepatitis C in Western China
- PMID: 30637215
- PMCID: PMC6328727
- DOI: 10.14218/JCTH.2018.00027
Large Disparity between Prevalence and Treatment Rates for Hepatitis C in Western China
Abstract
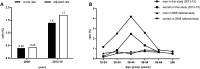
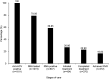
Background and Aims: Recently, the World Health Organization adopted the first-ever global hepatitis strategy with the dream of eliminating viral hepatitis as a public health threat by 2030. However, the epidemiology and treatment rates of hepatitis C virus (HCV) infection in Western China are still unknown. Methods: A total of 111,916 adult individuals (15-96 years) who underwent the HCV-antibody (HCV-Ab) test in the Second Affiliated Hospital of Chongqing Medical University between 2013 and 2015 were included in this study. We retrospectively analyzed the electronic medical records' data for each, and the positivity of HCV-Ab and the treatment of HCV RNA-positive patients were evaluated. Results: During 2013-2015, the crude prevalence of HCV-Ab was 1.4% (95%CI: 1.4-1.5; 1,611/111,916) and the adjusted prevalence of HCV-Ab was 1.7% (95%CI: 1.6-1.8), which was higher than in the 2006 national study (0.43%). The prevalence was 2-times higher in males than females (2.0% vs. 1.1%, p < 0.01). Notably, only 46% (434/951) of the HCV RNA-positive patients received standard peg-interferon plus ribavirin treatment, with 370 (82%) that completed treatment, of whom 272 (74%) achieved sustained virologic response (SVR). Particularly, 11% (32/292) of HCV RNA-positive patients were HBsAg-positive, and the SVR rate for them was lower than for the HBsAg-negative patients, but no significant difference was observed. Conclusions: HCV infection may have increased since 2006 in Western China. The SVR rate of peg-interferon plus ribavirin treatment was high, but the proportion of untreated HCV patients was large. Thus, more efforts need to be made by the government to create a scientific-based policy for HCV treatment and prevention.
Keywords: Epidemiology; Hepatitis C virus; Hospital-based population study; Treatment.
Conflict of interest statement
The authors have no conflict of interests related to this publication.
Figures

Similar articles
-
The treatment outcome and impact on blood transfusion demand of Peg-interferon/ribavirin in thalassemic patients with chronic hepatitis C.J Formos Med Assoc. 2018 Jan;117(1):14-23. doi: 10.1016/j.jfma.2017.10.001. Epub 2017 Oct 31. J Formos Med Assoc. 2018. PMID: 29097076
-
Treatment outcomes of patients with chronic hepatitis C receiving sofosbuvir-based combination therapy within national hepatitis C elimination program in the country of Georgia.BMC Infect Dis. 2020 Jan 10;20(1):30. doi: 10.1186/s12879-019-4741-5. BMC Infect Dis. 2020. PMID: 31924172 Free PMC article.
-
OPERA: responses to peginterferon and ribavirin therapy in a subgroup of interferon-naïve patients with HIV/HCV genotype 2/3 co-infection in Italy.Liver Int. 2015 Jan;35(1):120-9. doi: 10.1111/liv.12641. Epub 2014 Aug 11. Liver Int. 2015. PMID: 25041136 Clinical Trial.
-
[Hepatitis C--treatment of untreated (naive) patients].Acta Med Croatica. 2005;59(5):453-61. Acta Med Croatica. 2005. PMID: 16381243 Review. Croatian.
-
Inclusion of hepatitis C virus testing in National Health Screening to accelerate HCV elimination in South Korea.Glob Health Med. 2021 Oct 31;3(5):288-292. doi: 10.35772/ghm.2021.01057. Glob Health Med. 2021. PMID: 34782871 Free PMC article. Review.
Cited by
-
Identification of a New HCV Subtype 6xg Among Injection Drug Users in Kachin, Myanmar.Front Microbiol. 2019 Apr 18;10:814. doi: 10.3389/fmicb.2019.00814. eCollection 2019. Front Microbiol. 2019. PMID: 31057519 Free PMC article.
-
Provincial heterogeneity in the management of care cascade for hypertension, diabetes, and dyslipidaemia in China: Analysis of nationally representative population-based survey.Front Cardiovasc Med. 2022 Aug 23;9:923249. doi: 10.3389/fcvm.2022.923249. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36093142 Free PMC article.
-
Epidemiological characteristics and treatment challenges of chronic hepatitis C in the kashi region of xinjiang china: A retrospective investigation from 2018 to 2022.Sci Rep. 2025 Mar 21;15(1):9726. doi: 10.1038/s41598-025-94626-6. Sci Rep. 2025. PMID: 40118953 Free PMC article.
-
Plasma Inflammatory Biomarkers Associated with Advanced Liver Fibrosis in HIV-HCV-Coinfected Individuals.Int J Environ Res Public Health. 2020 Dec 17;17(24):9474. doi: 10.3390/ijerph17249474. Int J Environ Res Public Health. 2020. PMID: 33348839 Free PMC article.
-
Direct-acting Antiviral Regimens for Patients with Chronic Infection of Hepatitis C Virus Genotype 3 in China.J Clin Transl Hepatol. 2021 Jun 28;9(3):419-427. doi: 10.14218/JCTH.2020.00097. Epub 2021 May 12. J Clin Transl Hepatol. 2021. PMID: 34221928 Free PMC article. Review.
References
-
- World Health Organization Hepatitis C. Available from: http://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c , accessed June, 2018.
-
- World Health Organization Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection: Updated version, April 2016. Available from: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en . - PubMed
LinkOut - more resources
Full Text Sources
