Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics
- PMID: 30637364
- PMCID: PMC6329466
- DOI: 10.1200/PO.18.00183
Analysis of NTRK Alterations in Pan-Cancer Adult and Pediatric Malignancies: Implications for NTRK-Targeted Therapeutics
Abstract
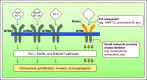
Purpose: Fusions that involve neurotrophic-tropomyosin receptor kinase (NTRK) genes are known drivers of oncogenesis. Therapies that target these ultra-rare, constitutionally active NTRK fusions have been remarkably effective. Herein, we analyze the prevalence of the full array of NTRK alterations-fusions, mutations, copy number alterations, and increased transcript expression-in diverse adult and pediatric tumor types to understand the landscape of NTRK aberrations in cancer.
Methods: We assessed 13,467 samples available from The Cancer Genome Atlas (adult tumors) and the St Jude PeCan database (pediatric tumors) for the prevalence of NTRK fusions, as well as associated genomic and transcriptomic co-aberrations in different tumor types.
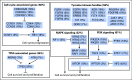
Results: NTRK fusions were observed in 0.31% of adult tumors and in 0.34% of pediatric tumors. The most common gene partners were NTRK3 (0.16% of adult tumors) followed by NTRK1 (0.14% of pediatric tumors). NTRK fusions were found more commonly in pediatric melanoma (11.1% of samples), pediatric glioma (3.97%), and adult thyroid cancers (2.34%). Additional genomic and transcriptomic NTRK alterations- mutation, amplification, and mRNA overexpression-occurred in 14.2% of samples, whereas the frequency of alterations that implicated NTRK ligands and the NTRK co-receptor (p75NTR) ranged from 3.8% to 5.4%. Among 31 adult samples carrying NTRK fusions, co-alterations occurred often and usually involved the downstream phosphoinositide-3-kinase signaling pathway, cell-cycle machinery, other tyrosine-kinase receptors, and mitogen-activated protein kinase signals.
Conclusion: Whereas NTRK fusions are exceedingly rare, other NTRK abnormalities affect 14% of patients with cancer. Affecting these alterations has not yet been achievable in cancer. Genomic co-alterations occur frequently with NTRK fusions, but it is not known if co-targeting them can attenuate primary or secondary resistance to NTRK inhibitors.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted.
Figures



References
-
- Segal RA. Selectivity in neurotrophin signaling: Theme and variations. Annu Rev Neurosci. 2003;26:299–330. - PubMed
-
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991;350:158–160. - PubMed
-
- Kawamura K, Kawamura N, Fukuda J, et al. Regulation of preimplantation embryo development by brain-derived neurotrophic factor. Dev Biol. 2007;311:147–158. - PubMed
-
- Kawamura K, Kawamura N, Kumazawa Y, et al. Brain-derived neurotrophic factor/tyrosine kinase B signaling regulates human trophoblast growth in an in vivo animal model of ectopic pregnancy. Endocrinology. 2011;152:1090–1100. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

