Elevated Sera sST2 Is Associated With Heart Failure in Men ≤50 Years Old With Myocarditis
- PMID: 30638108
- PMCID: PMC6497352
- DOI: 10.1161/JAHA.118.008968
Elevated Sera sST2 Is Associated With Heart Failure in Men ≤50 Years Old With Myocarditis
Abstract
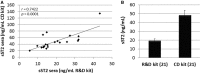
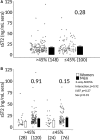
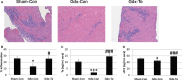
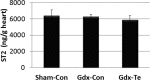
Background Myocarditis is an important cause of acute and chronic heart failure. Men with myocarditis have worse recovery and an increased need for transplantation compared with women, but the reason for the sex difference remains unclear. Elevated sera soluble (s) ST2 predicts mortality from acute and chronic heart failure, but has not been studied in myocarditis patients. Methods and Results Adults with a diagnosis of clinically suspected myocarditis (n=303, 78% male) were identified according to the 2013 European Society of Cardiology position statement. Sera sST2 levels were examined by ELISA in humans and mice and correlated with heart function according to sex and age. Sera sST2 levels were higher in healthy men ( P=8×10-6) and men with myocarditis ( P=0.004) compared with women. sST2 levels were elevated in patients with myocarditis and New York Heart Association class III - IV heart failure ( P=0.002), predominantly in men ( P=0.0003). Sera sST2 levels were associated with New York Heart Association class in men with myocarditis who were ≤50 years old ( r=0.231, P=0.0006), but not in women ( r=0.172, P=0.57). Sera sST2 levels were also significantly higher in male mice with myocarditis ( P=0.005) where levels were associated with cardiac inflammation. Gonadectomy with hormone replacement showed that testosterone ( P<0.001), but not estradiol ( P=0.32), increased sera sST2 levels in male mice with myocarditis. Conclusions We show in a well-characterized subset of heart failure patients with clinically suspected and biopsy-confirmed myocarditis that elevated sera sST2 is associated with an increased risk of heart failure based on New York Heart Association class in men ≤50 years old.
Keywords: biomarkers; heart failure; myocarditis; sST2; sex differences.
Figures
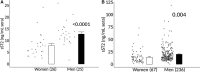














References
-
- Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. - PubMed
-
- Cooper LT Jr, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014;9:121–129. - PubMed
-
- Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

