Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study
- PMID: 30638863
- PMCID: PMC6412085
- DOI: 10.1016/j.ebiom.2018.12.051
Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study
Abstract
Background: VIS410, a broadly neutralizing monoclonal antibody that binds the hemagglutinin stem of influenza A viruses, was safe and efficacious in a human H1N1 virus challenge study. This study evaluated the safety and tolerability of VIS410 in non-hospitalized adult patients with uncomplicated influenza A.
Methods: Patients 18 to 65 years of age with symptom onset within 72 h were randomized 1:1:1 to receive a single intravenous infusion of VIS410 4000 mg, 2000 mg, or placebo. Neuraminidase inhibitor therapy was prohibited. Treatment-emergent adverse events (TEAEs) were evaluated up to 100 days post-infusion. Influenza symptoms were assessed daily for 10 days using the FLU-PRO tool. Nasopharyngeal virus shedding was assessed by quantitative reverse-transcription PCR (qRT-PCR) and viral culture through Day 7.
Findings: Of the 150 patients randomized, 148 received study drug, and 138 were confirmed influenza A positive. Median age was 42 years; median time from symptom onset to treatment was 42 h; 93% had influenza A subtype H3N2.
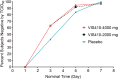
Safety: TEAEs, most commonly diarrhea of mild severity, were dose-related, occurring in 55%, 35%, and 24% of the 4000 mg, 2000 mg, and placebo patients, respectively. Two serious adverse events occurred, both in placebo patients.
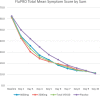
Symptom analyses: Baseline FLU-PRO symptom scores were balanced among groups. Mean scores were lower by Days 3 and 4 in the pooled VIS410 treatment group versus placebo (p < 0.023), with a tendency toward faster resolution by Kaplan-Meier analysis.
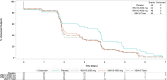
Virology analyses: VIS410 was associated with reduced median nasopharyngeal viral load TCID50 AUCDay7 (days × log10 TCID50/mL) (3.66 pooled VIS410 vs 4.78 placebo, p = 0.08) and in the subset of patients with baseline hemagglutination inhibition (HAI) titer ≤40 (overall, 74% of patients) was significantly reduced vs placebo (4.218 pooled VIS410 vs 6.152 placebo, p = 0.009). Kaplan-Meier estimated time to resolution of viral shedding was reduced (1.9 vs 3.6 days, p = 0.03) in VIS410 treated patients. There was a trend toward greater proportion of culture-negative patients by Day 3 (66.7% vs 51.1%, p = 0.11); when this analysis was limited to the subset of patients with positive baseline cultures, this difference became more pronounced (63.2% vs 42.5%, p = 0.053). No differences were observed in nasopharyngeal influenza qRT-PCR profiles, which represent both live and neutralized virus.
Interpretation: VIS410 was safe and well tolerated in adults with uncomplicated influenza A, with favorable effects on symptom resolution and virus replication.
Trial registration: Clinical Trials: NCT02989194.
Funding: This project was funded in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under Contract No. HHSO100201500018C.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Clinical and virological responses to a broad-spectrum human monoclonal antibody in an influenza virus challenge study.Antiviral Res. 2020 Dec;184:104763. doi: 10.1016/j.antiviral.2020.104763. Epub 2020 Mar 7. Antiviral Res. 2020. PMID: 32151645 Clinical Trial.
-
Safety and Upper Respiratory Pharmacokinetics of the Hemagglutinin Stalk-Binding Antibody VIS410 Support Treatment and Prophylaxis Based on Population Modeling of Seasonal Influenza A Outbreaks.EBioMedicine. 2016 Feb 26;5:147-55. doi: 10.1016/j.ebiom.2016.02.021. eCollection 2016 Mar. EBioMedicine. 2016. PMID: 27077121 Free PMC article. Clinical Trial.
-
Phase 2 Randomized Trial of the Safety and Efficacy of MHAA4549A, a Broadly Neutralizing Monoclonal Antibody, in a Human Influenza A Virus Challenge Model.Antimicrob Agents Chemother. 2017 Oct 24;61(11):e01154-17. doi: 10.1128/AAC.01154-17. Print 2017 Nov. Antimicrob Agents Chemother. 2017. PMID: 28807912 Free PMC article. Clinical Trial.
-
Tackling influenza with broadly neutralizing antibodies.Curr Opin Virol. 2017 Jun;24:60-69. doi: 10.1016/j.coviro.2017.03.002. Epub 2017 May 18. Curr Opin Virol. 2017. PMID: 28527859 Free PMC article. Review.
-
Broad-range neutralizing anti-influenza A human monoclonal antibodies: new perspectives in therapy and prophylaxis.New Microbiol. 2012 Oct;35(4):399-406. Epub 2012 Oct 1. New Microbiol. 2012. PMID: 23109007 Review.
Cited by
-
Respiratory Viruses in Solid Organ Transplant Recipients.Viruses. 2021 Oct 25;13(11):2146. doi: 10.3390/v13112146. Viruses. 2021. PMID: 34834953 Free PMC article. Review.
-
Structural Biology Illuminates Molecular Determinants of Broad Ebolavirus Neutralization by Human Antibodies for Pan-Ebolavirus Therapeutic Development.Front Immunol. 2022 Jan 10;12:808047. doi: 10.3389/fimmu.2021.808047. eCollection 2021. Front Immunol. 2022. PMID: 35082794 Free PMC article. Review.
-
Drug combination therapy for emerging viral diseases.Drug Discov Today. 2021 Oct;26(10):2367-2376. doi: 10.1016/j.drudis.2021.05.008. Epub 2021 May 21. Drug Discov Today. 2021. PMID: 34023496 Free PMC article. Review.
-
Recent Progress in the Discovery and Development of Monoclonal Antibodies against Viral Infections.Biomedicines. 2022 Aug 2;10(8):1861. doi: 10.3390/biomedicines10081861. Biomedicines. 2022. PMID: 36009408 Free PMC article. Review.
-
Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy.Adv Drug Deliv Rev. 2021 Feb;169:100-117. doi: 10.1016/j.addr.2020.12.004. Epub 2020 Dec 9. Adv Drug Deliv Rev. 2021. PMID: 33309815 Free PMC article. Review.
References
-
- WHO . Feb. 2010. Guidelines for pharmacological management of Pandemic influenza A(H1N1) 2009 and other influenza viruses. Revised. - PubMed
-
- Hayden F.G., Sugaya N., Hirotsu N. Baloxavir Marboxil for Uncomplicated Influenza in adults and Adolescents. N Engl J Med. 2018;379(10):913–923. - PubMed
-
- Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

