Automatic Detection and Classification of Ca2+ Release Events in Line- and Frame-Scan Images
- PMID: 30638961
- PMCID: PMC6369403
- DOI: 10.1016/j.bpj.2018.12.013
Automatic Detection and Classification of Ca2+ Release Events in Line- and Frame-Scan Images
Abstract
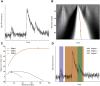
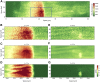
Analysis of Ca2+ signals obtained in various cell types (i.e., cardiomyocytes) is always a tradeoff between acquisition speed and signal/noise ratio of the fluorescence signal. This becomes especially apparent during fast two- or three-dimensional confocal imaging when local intracellular fluorescence signals originating from Ca2+ release from intracellular Ca2+ stores (e.g., sarcoplasmic reticulum) need to be examined. Mathematical methods have been developed to remedy a high noise level by fitting each pixel with a transient function to "denoise" the image. So far, current available analytical approaches have been impaired by a number of constraints (e.g., inability to fit local, concurrent, and consecutive events) and the limited ability to customize implementation. Here, we suggest a, to our knowledge, novel approach for detailed analysis of subcellular micro-Ca2+ events based on pixel-by-pixel denoising of confocal frame- and line-scan images. The algorithm enables spatiotemporally overlapping events (e.g., a Ca2+ spark occurring during the decaying phase of a Ca2+ wave) to be extracted so that various types of Ca2+ events can be detected at a pixel time level of precision. The method allows a nonconstant baseline to be estimated for each pixel, foregoing the need to subtract fluorescence background or apply self-ratio methods before image analysis. Furthermore, by using a clustering algorithm, identified single-pixel events are grouped into "physiologically relevant" Ca2+ signaling events spanning multiple pixels (sparks, waves, puffs, transients, etc.), from which spatiotemporal event parameters (e.g., full duration at half maximal amplitude, full width at half maximal amplitude, amplitude, wave speed, rise, and decay times) can be easily extracted. The method was implemented with cross-platform open source software, providing a comprehensive and easy-to-use graphical user interface enabling rapid line-scan images and rapid frame-scan image sequences (up to 150 frames/s) to be analyzed and repetitive Ca2+ events (Ca2+ sparks and Ca2+ puffs) originating from clusters of Ca2+ release channels located in the sarcoplasmic reticulum membrane (ryanodine receptors and inositol 1,4,5-trisphosphate receptors) of isolated cardiomyocytes to be examined with a high level of precision.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Niggli E. Localized intracellular calcium signaling in muscle: calcium sparks and calcium quarks. Annu. Rev. Physiol. 1999;61:311–335. - PubMed
-
- Niggli E., Shirokova N. A guide to sparkology: the taxonomy of elementary cellular Ca2+ signaling events. Cell Calcium. 2007;42:379–387. - PubMed
-
- Uttenweiler D., Kirsch W.G., Fink R.H. Model-based analysis of elementary Ca2+ release events in skinned mammalian skeletal muscle fibres. Eur. Biophys. J. 2002;31:331–340. - PubMed
-
- Picht E., Zima A.V., Bers D.M. SparkMaster: automated calcium spark analysis with ImageJ. Am. J. Physiol. Cell Physiol. 2007;293:C1073–C1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

