Rickettsia Sca2 Recruits Two Actin Subunits for Nucleation but Lacks WH2 Domains
- PMID: 30638962
- PMCID: PMC6369429
- DOI: 10.1016/j.bpj.2018.12.009
Rickettsia Sca2 Recruits Two Actin Subunits for Nucleation but Lacks WH2 Domains
Abstract
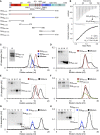
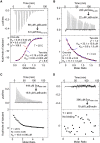
The Rickettsia ∼1800-amino-acid autotransporter protein surface cell antigen 2 (Sca2) promotes actin polymerization on the surface of the bacterium to drive its movement using an actin comet-tail mechanism. Sca2 mimics eukaryotic formins in that it promotes both actin filament nucleation and elongation and competes with capping protein to generate filaments that are long and unbranched. However, despite these functional similarities, Sca2 is structurally unrelated to eukaryotic formins and achieves these functions through an entirely different mechanism. Thus, while formins are dimeric, Sca2 functions as a monomer. However, Sca2 displays intramolecular interactions and functional cooperativity between its N- and C-terminal domains that are crucial for actin nucleation and elongation. Here, we map the interaction of N- and C- terminal fragments of Sca2 and their contribution to actin binding and nucleation. We find that both the N- and C-terminal regions of Sca2 interact with actin monomers but only weakly, whereas the full-length protein binds two actin monomers with high affinity. Moreover, deletions at both ends of the N- and C-terminal regions disrupt their ability to interact with each other, suggesting that they form a contiguous ring-like structure that wraps around two actin subunits, analogous to the formin homology-2 domain. The discovery of Sca2 as an actin nucleator followed the identification of what appeared to be a repeat of three Wiskott-Aldrich syndrome homology 2 (WH2) domains in the middle of the molecule, consistent with the presence of WH2 domains in most actin nucleators. However, we show here that contrary to previous assumptions, Sca2 does not contain WH2 domains. Instead, our analysis indicates that the region containing the putative WH2 domains is folded as a globular domain that cooperates with other parts of the Sca2 molecule for actin binding and nucleation.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Truong D., Copeland J.W., Brumell J.H. Bacterial subversion of host cytoskeletal machinery: hijacking formins and the Arp2/3 complex. BioEssays. 2014;36:687–696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

