Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex
- PMID: 30639101
- PMCID: PMC6461403
- DOI: 10.1016/j.cell.2018.11.040
Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex
Abstract
Cannabis elicits its mood-enhancing and analgesic effects through the cannabinoid receptor 1 (CB1), a G protein-coupled receptor (GPCR) that signals primarily through the adenylyl cyclase-inhibiting heterotrimeric G protein Gi. Activation of CB1-Gi signaling pathways holds potential for treating a number of neurological disorders and is thus crucial to understand the mechanism of Gi activation by CB1. Here, we present the structure of the CB1-Gi signaling complex bound to the highly potent agonist MDMB-Fubinaca (FUB), a recently emerged illicit synthetic cannabinoid infused in street drugs that have been associated with numerous overdoses and fatalities. The structure illustrates how FUB stabilizes the receptor in an active state to facilitate nucleotide exchange in Gi. The results compose the structural framework to explain CB1 activation by different classes of ligands and provide insights into the G protein coupling and selectivity mechanisms adopted by the receptor.
Keywords: CB1; Fubinaca; G(i); GPCR; cannabinoid receptor; cryo-EM; synthetic cannabinoid.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
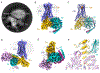
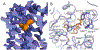




Comment in
-
Receptor Structures for a Caldron of Cannabinoids.Cell. 2019 Jan 24;176(3):409-411. doi: 10.1016/j.cell.2019.01.012. Cell. 2019. PMID: 30682366
References
-
- Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, and Gerona R (2017). “Zombie” Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med 376, 235–242. - PubMed
-
- Ballesteros JA, and Weinstein H (1995). Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. In Methods in Neurosciences, (Elsevier), pp. 366–428.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

