Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson's Disease
- PMID: 30639209
- PMCID: PMC6372974
- DOI: 10.1016/j.stemcr.2018.12.011
Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson's Disease
Abstract
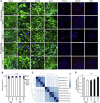
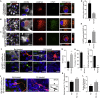
Parkinson's disease (PD) is associated with the degeneration of ventral midbrain dopaminergic neurons (vmDAns) and the accumulation of toxic α-synuclein. A non-cell-autonomous contribution, in particular of astrocytes, during PD pathogenesis has been suggested by observational studies, but remains to be experimentally tested. Here, we generated induced pluripotent stem cell-derived astrocytes and neurons from familial mutant LRRK2 G2019S PD patients and healthy individuals. Upon co-culture on top of PD astrocytes, control vmDAns displayed morphological signs of neurodegeneration and abnormal, astrocyte-derived α-synuclein accumulation. Conversely, control astrocytes partially prevented the appearance of disease-related phenotypes in PD vmDAns. We additionally identified dysfunctional chaperone-mediated autophagy (CMA), impaired macroautophagy, and progressive α-synuclein accumulation in PD astrocytes. Finally, chemical enhancement of CMA protected PD astrocytes and vmDAns via the clearance of α-synuclein accumulation. Our findings unveil a crucial non-cell-autonomous contribution of astrocytes during PD pathogenesis, and open the path to exploring novel therapeutic strategies aimed at blocking the pathogenic cross talk between neurons and glial cells.
Keywords: CRISPR/Cas9; LRRK2; Parkinson's disease; astrocytes; autophagy; disease modeling; iPSC; neurodegeneration; non-cell-autonomous; α-synuclein.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Abeliovich A., Gitler A.D. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature. 2016;539:207–216. - PubMed
-
- Alwin S., Gere M.B., Guhl E., Effertz K., Barbas C.F., 3rd, Segal D.J., Weitzman M.D., Cathomen T. Custom zinc-finger nucleases for use in human cells. Mol. Ther. 2005;12:610–617. - PubMed
-
- Braak H., Sastre M., Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol. 2007;114:231–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

