Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications
- PMID: 30639256
- PMCID: PMC7115878
- DOI: 10.1016/j.addr.2019.01.005
Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications
Abstract
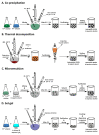
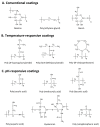
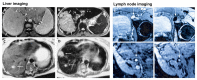
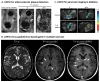
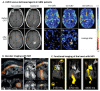
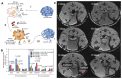
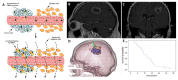
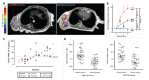
Many different iron oxide nanoparticles have been evaluated over the years, for a wide variety of biomedical applications. We here summarize the synthesis, surface functionalization and characterization of iron oxide nanoparticles, as well as their (pre-) clinical use in diagnostic, therapeutic and theranostic settings. Diagnostic applications include liver, lymph node, inflammation and vascular imaging, employing mostly magnetic resonance imaging but recently also magnetic particle imaging. Therapeutic applications encompass iron supplementation in anemia and advanced cancer treatments, such as modulation of macrophage polarization, magnetic fluid hyperthermia and magnetic drug targeting. Because of their properties, iron oxide nanoparticles are particularly useful for theranostic purposes. Examples of such setups, in which diagnosis and therapy are intimately combined and in which iron oxide nanoparticles are used, are image-guided drug delivery, image-guided and microbubble-mediated opening of the blood-brain barrier, and theranostic tissue engineering. Together, these directions highlight the versatility and the broad applicability of iron oxide nanoparticles, and indicate the integration in future medical practice of multiple iron oxide nanoparticle-based materials.
Keywords: MFH; MPI; MRI; Nanomedicine; SPION; Theranostics; USPIO.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
References
-
- Figuerola A, Di Corato R, Manna L, Pellegrino T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res. 2010;62:126–143. - PubMed
-
- Mohammed L, Gomaa HG, Ragab D, Zhu J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology. 2017;30:1–14.
-
- Jin R, Lin B, Li D, Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol. 2014;18:18–27. - PubMed
-
- Cheng F-Y, Su C-H, Yang Y-S, Yeh C-S, Tsai C-Y, Wu C-L, Wu M-T, Shieh D-B. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26:729–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

