Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial
- PMID: 30640362
- PMCID: PMC6459135
- DOI: 10.1001/jamaneurol.2018.4304
Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial
Erratum in
-
Error in Byline.JAMA Neurol. 2019 Apr 1;76(4):509. doi: 10.1001/jamaneurol.2019.0575. JAMA Neurol. 2019. PMID: 30958554 Free PMC article. No abstract available.
Abstract
Importance: Exenatide, a glucagon-like peptide 1 agonist used in type 2 diabetes, was recently found to have beneficial effects on motor function in a randomized, placebo-controlled trial in Parkinson disease (PD). Accumulating evidence suggests that impaired brain insulin and protein kinase B (Akt) signaling play a role in PD pathogenesis; however, exploring the extent to which drugs engage with putative mechnisms in vivo remains a challenge.
Objective: To assess whether participants in the Exenatide-PD trial have augmented activity in brain insulin and Akt signaling pathways.
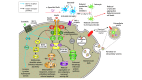
Design, setting, and participants: Serum samples were collected from 60 participants in the single-center Exenatide-PD trial (June 18, 2014, to June 16, 2016), which compared patients with moderate PD randomized to 2 mg of exenatide once weekly or placebo for 48 weeks followed by a 12-week washout period. Serum extracellular vesicles, including exosomes, were extracted, precipitated, and enriched for neuronal source by anti-L1 cell adhesion molecule antibody absorption, and proteins of interest were evaluated using electrochemiluminescence assays. Statistical analysis was performed from May 1, 2017, to August 31, 2017.
Main outcomes and measures: The main outcome was augmented brain insulin signaling that manifested as a change in tyrosine phosphorylated insulin receptor substrate 1 within neuronal extracellular vesicles at the end of 48 weeks of exenatide treatment. Additional outcome measures were changes in other insulin receptor substrate proteins and effects on protein expression in the Akt and mitogen-activated protein kinase pathways.
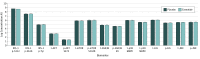
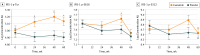
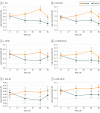
Results: Sixty patients (mean [SD] age, 59.9 [8.4] years; 43 [72%] male) participated in the study: 31 in the exenatide group and 29 in the placebo group (data from 1 patient in the exenatide group were excluded). Patients treated with exenatide had augmented tyrosine phosphorylation of insulin receptor substrate 1 at 48 weeks (0.27 absorbance units [AU]; 95% CI, 0.09-0.44 AU; P = .003) and 60 weeks (0.23 AU; 95% CI, 0.05-0.41 AU; P = .01) compared with patients receiving placebo. Exenatide-treated patients had elevated expression of downstream substrates, including total Akt (0.35 U/mL; 95% CI, 0.16-0.53 U/mL; P < .001) and phosphorylated mechanistic target of rapamycin (mTOR) (0.22 AU; 95% CI, 0.04-0.40 AU; P = .02). Improvements in Movement Disorders Society Unified Parkinson's Disease Rating Scale part 3 off-medication scores were associated with levels of total mTOR (F4,50 = 5.343, P = .001) and phosphorylated mTOR (F4,50 = 4.384, P = .04).
Conclusions and relevance: The results of this study are consistent with target engagement of brain insulin, Akt, and mTOR signaling pathways by exenatide and provide a mechanistic context for the clinical findings of the Exenatide-PD trial. This study suggests the potential of using exosome-based biomarkers as objective measures of target engagement in clinical trials using drugs that target neuronal pathways.
Conflict of interest statement
Figures

Comment in
-
Neural-Derived Extracellular Vesicles in Clinical Trials: Message in a Bottle.JAMA Neurol. 2019 Apr 1;76(4):402-404. doi: 10.1001/jamaneurol.2018.4325. JAMA Neurol. 2019. PMID: 30640380 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

