Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance
- PMID: 30640366
- PMCID: PMC6439883
- DOI: 10.1001/jamapediatrics.2018.4826
Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance
Erratum in
-
Errors in Byline, Abstract, and Discussion.JAMA Pediatr. 2019 Mar 1;173(3):296. doi: 10.1001/jamapediatrics.2019.0061. JAMA Pediatr. 2019. PMID: 30830159 Free PMC article. No abstract available.
-
Error in Results.JAMA Pediatr. 2019 May 1;173(5):502. doi: 10.1001/jamapediatrics.2019.0953. JAMA Pediatr. 2019. PMID: 30985896 Free PMC article. No abstract available.
Abstract
Importance: Invasive disease owing to group B Streptococcus (GBS) remains an important cause of illness and death among infants younger than 90 days in the United States, despite declines in early-onset disease (EOD; with onset at 0-6 days of life) that are attributed to intrapartum antibiotic prophylaxis (IAP). Maternal vaccines to prevent infant GBS disease are currently under development.
Objective: To describe incidence rates, case characteristics, antimicrobial resistance, and serotype distribution of EOD and late-onset disease (LOD; with onset at 7-89 days of life) in the United States from 2006 to 2015 to inform IAP guidelines and vaccine development.
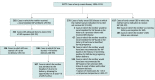
Design, setting, and participants: This study used active population-based and laboratory-based surveillance for invasive GBS disease conducted through Active Bacterial Core surveillance in selected counties of 10 states across the United States. Residents of Active Bacterial Core surveillance areas who were younger than 90 days and had invasive GBS disease in 2006 to 2015 were included. Data were analyzed from December 2017 to April 2018.
Exposures: Group B Streptococcus isolated from a normally sterile site.
Main outcomes and measures: Early-onset disease and LOD incidence rates and associated GBS serotypes and antimicrobial resistance.
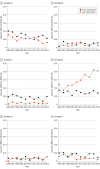
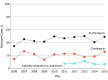
Results: The Active Bacterial Core surveillance program identified 1277 cases of EOD and 1387 cases of LOD. From 2006 to 2015, EOD incidence declined significantly from 0.37 to 0.23 per 1000 live births (P < .001), and LOD rates remained stable (mean, 0.31 per 1000 live births). Among the mothers of 1277 infants with EOD, 617 (48.3%) had no indications for IAP and did not receive it, and 278 (21.8%) failed to receive IAP despite having indications. Serotype data were available for 1743 of 1897 patients (91.3%) from 7 sites that collect GBS isolates. Among patients with EOD, serotypes Ia (242 [27.3%]) and III (242 [27.3%]) were most common. Among patients with LOD, serotype III was most common (481 [56.2%]), and this increased from 2006 to 2015 from 0.12 to 0.20 cases per 1000 live births (P < .001). Serotype IV caused 53 cases (6.2%) of EOD and LOD combined. The 6 most common serotypes (Ia, Ib, II, III, IV, and V) caused 881 EOD cases (99.3%) and 853 LOD cases (99.7%). No β-lactam resistance was identified; 359 isolates (20.8%) tested showed constitutive clindamycin resistance. In 2015, an estimated 840 EOD cases and 1265 LOD cases occurred nationally.
Conclusions and relevance: The rates of LOD among US infants are now higher than EOD rates. Combined with addressing IAP implementation gaps, an effective vaccine covering the most common serotypes might further reduce EOD rates and help prevent LOD, for which there is no current public health intervention.
Conflict of interest statement
Figures

Comment in
-
Preventing Neonatal Group B Streptococcus Disease: The Limits of Success.JAMA Pediatr. 2019 Mar 1;173(3):219-220. doi: 10.1001/jamapediatrics.2018.4824. JAMA Pediatr. 2019. PMID: 30640370 No abstract available.
References
-
- Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31(suppl 4):D20-D26. doi: 10.1016/j.vaccine.2012.11.056 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24. - PubMed
-
- Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22. - PubMed
-
- Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) . Prevention of perinatal group B streptococcal disease—revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59(RR-10):1-36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

