Complication Rates and Downstream Medical Costs Associated With Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting
- PMID: 30640382
- PMCID: PMC6440230
- DOI: 10.1001/jamainternmed.2018.6277
Complication Rates and Downstream Medical Costs Associated With Invasive Diagnostic Procedures for Lung Abnormalities in the Community Setting
Abstract
Importance: The Centers for Medicare & Medicaid Services added lung cancer screening with low-dose computed tomography (LDCT) as a Medicare preventive service benefit in 2015 following findings from the National Lung Screening Trial (NLST) that showed a 16% reduction in lung cancer mortality associated with LDCT. A challenge in developing and promoting a national lung cancer screening program is the high false-positive rate of LDCT because abnormal findings from thoracic imaging often trigger subsequent invasive diagnostic procedures and could lead to postprocedural complications.
Objective: To determine the complication rates and downstream medical costs associated with invasive diagnostic procedures performed for identification of lung abnormalities in the community setting.
Design, setting, and participants: A retrospective cohort study of non-protocol-driven community practices captured in MarketScan Commercial Claims & Encounters and Medicare supplemental databases was conducted. A nationally representative sample of 344 510 patients aged 55 to 77 years who underwent invasive diagnostic procedures between 2008 and 2013 was included.
Main outcomes and measures: One-year complication rates were calculated for 4 groups of invasive diagnostic procedures. The complication rates and costs were further stratified by age group.
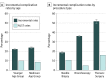
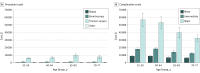
Results: Of the 344 510 individuals aged 55 to 77 years included in the study, 174 702 comprised the study group (109 363 [62.6%] women) and 169 808 served as the control group (106 007 [62.4%] women). The estimated complication rate was 22.2% (95% CI, 21.7%-22.7%) for individuals in the young age group and 23.8% (95% CI, 23.0%-24.6%) for those in the Medicare group; the rates were approximately twice as high as those reported in the NLST (9.8% and 8.5%, respectively). The mean incremental complication costs were $6320 (95% CI, $5863-$6777) for minor complications to $56 845 (95% CI, $47 953-$65 737) for major complications.
Conclusions and relevance: The rates of complications after invasive diagnostic procedures were higher than the rates reported in clinical trials. Physicians and patients should be aware of the potential risks of subsequent adverse events and their high downstream costs in the shared decision-making process.
Conflict of interest statement
Figures
Comment in
-
Complication Rates in a Study of Invasive Diagnostic Procedures for Lung Abnormalities.JAMA Intern Med. 2019 Jun 1;179(6):846-847. doi: 10.1001/jamainternmed.2019.0960. JAMA Intern Med. 2019. PMID: 31157840 No abstract available.
-
Complication Rates in a Study of Invasive Diagnostic Procedures for Lung Abnormalities-In Reply.JAMA Intern Med. 2019 Jun 1;179(6):847. doi: 10.1001/jamainternmed.2019.0954. JAMA Intern Med. 2019. PMID: 31157849 No abstract available.
Similar articles
-
Use of Imaging and Diagnostic Procedures After Low-Dose CT Screening for Lung Cancer.Chest. 2020 Feb;157(2):427-434. doi: 10.1016/j.chest.2019.08.2187. Epub 2019 Sep 12. Chest. 2020. PMID: 31521671 Free PMC article.
-
Patient-Level Trajectories and Outcomes After Low-Dose CT Screening in the National Lung Screening Trial.Chest. 2019 Nov;156(5):965-971. doi: 10.1016/j.chest.2019.06.016. Epub 2019 Jul 5. Chest. 2019. PMID: 31283920 Clinical Trial.
-
Rates of Downstream Procedures and Complications Associated With Lung Cancer Screening in Routine Clinical Practice : A Retrospective Cohort Study.Ann Intern Med. 2024 Jan;177(1):18-28. doi: 10.7326/M23-0653. Epub 2024 Jan 2. Ann Intern Med. 2024. PMID: 38163370 Free PMC article.
-
[Diagnostic invasive procedures in the diagnosis of primary lung cancer. Diagnostic value and complications].Ugeskr Laeger. 1996 Dec 30;159(1):37-40. Ugeskr Laeger. 1996. PMID: 9012072 Review. Danish.
-
Low-Dose CT Scan for Lung Cancer Screening: Clinical and Coding Considerations.Chest. 2017 Jul;152(1):204-209. doi: 10.1016/j.chest.2017.03.019. Epub 2017 Mar 21. Chest. 2017. PMID: 28336485 Review.
Cited by
-
Downstream Complications and Healthcare Expenditure after Invasive Procedures for Lung Lesions in Taiwan.Int J Environ Res Public Health. 2021 Apr 12;18(8):4040. doi: 10.3390/ijerph18084040. Int J Environ Res Public Health. 2021. PMID: 33921313 Free PMC article.
-
Economic Evaluation of a Novel Lung Cancer Diagnostic in a Population of Patients with a Positive Low-Dose Computed Tomography Result.J Health Econ Outcomes Res. 2024 Sep 17;11(2):74-79. doi: 10.36469/001c.121512. eCollection 2024. J Health Econ Outcomes Res. 2024. PMID: 39810799 Free PMC article.
-
Lung cancer screening in people who have never smoked: lessons from East Asia.BMJ. 2025 Feb 6;388:e081674. doi: 10.1136/bmj-2024-081674. BMJ. 2025. PMID: 39914848 Free PMC article.
-
Real-World Genomic Landscape of Gastrointestinal Cancers in Asia and the Middle East Using Comprehensive Circulating Tumor DNA Next-Generation Sequencing.Oncol Res Treat. 2025 May 5:1-17. doi: 10.1159/000545560. Online ahead of print. Oncol Res Treat. 2025. PMID: 40324346 Free PMC article.
-
Liquid and Tissue Biopsies for Identifying MET Exon 14 Skipping NSCLC: Analyses from the Phase II VISION Study of Tepotinib.Clin Cancer Res. 2025 Jul 1;31(13):2675-2684. doi: 10.1158/1078-0432.CCR-24-4097. Clin Cancer Res. 2025. PMID: 40310449 Free PMC article. Clinical Trial.
References
-
- Howlader N, Noone A, Krapcho M, et al. . SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; 2014.

