Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement
- PMID: 30640517
- PMCID: PMC6835090
- DOI: 10.1164/rccm.201810-1895ST
Current Status and Future Opportunities in Lung Precision Medicine Research with a Focus on Biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement
Abstract
Background: Thousands of biomarker tests are either available or under development for lung diseases. In many cases, adoption of these tests into clinical practice is outpacing the generation and evaluation of sufficient data to determine clinical utility and ability to improve health outcomes. There is a need for a systematically organized report that provides guidance on how to understand and evaluate use of biomarker tests for lung diseases.
Methods: We assembled a diverse group of clinicians and researchers from the American Thoracic Society and leaders from the National Heart, Lung, and Blood Institute with expertise in various aspects of precision medicine to review the current status of biomarker tests in lung diseases. Experts summarized existing biomarker tests that are available for lung cancer, pulmonary arterial hypertension, idiopathic pulmonary fibrosis, asthma, chronic obstructive pulmonary disease, sepsis, acute respiratory distress syndrome, cystic fibrosis, and other rare lung diseases. The group identified knowledge gaps that future research studies can address to efficiently translate biomarker tests into clinical practice, assess their cost-effectiveness, and ensure they apply to diverse, real-life populations.
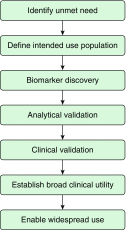
Results: We found that the status of biomarker tests in lung diseases is highly variable depending on the disease. Nevertheless, biomarker tests in lung diseases show great promise in improving clinical care. To efficiently translate biomarkers into tests used widely in clinical practice, researchers need to address specific clinical unmet needs, secure support for biomarker discovery efforts, conduct analytical and clinical validation studies, ensure tests have clinical utility, and facilitate appropriate adoption into routine clinical practice.
Conclusions: Although progress has been made toward implementation of precision medicine for lung diseases in clinical practice in certain settings, additional studies focused on addressing specific unmet clinical needs are required to evaluate the clinical utility of biomarkers; ensure their generalizability to diverse, real-life populations; and determine their cost-effectiveness.
Keywords: biomarker; precision medicine; pulmonary.
Comment in
-
Reply to Mahler: Peak Inspiratory Flow Rate: An Emerging Biomarker in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2019 Jun 15;199(12):1579. doi: 10.1164/rccm.201902-0432LE. Am J Respir Crit Care Med. 2019. PMID: 30892053 Free PMC article. No abstract available.
-
Peak Inspiratory Flow Rate: An Emerging Biomarker in Chronic Obstructive Pulmonary Disease.Am J Respir Crit Care Med. 2019 Jun 15;199(12):1577-1579. doi: 10.1164/rccm.201901-0005LE. Am J Respir Crit Care Med. 2019. PMID: 30892057 Free PMC article. No abstract available.
References
-
- McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med. 2013;5:189sr4. - PubMed
-
- Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical