The brown algal mode of tip growth: Keeping stress under control
- PMID: 30640903
- PMCID: PMC6347293
- DOI: 10.1371/journal.pbio.2005258
The brown algal mode of tip growth: Keeping stress under control
Abstract
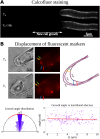
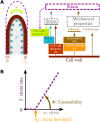
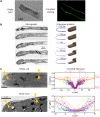
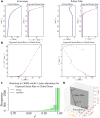
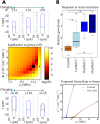
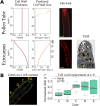
Tip growth has been studied in pollen tubes, root hairs, and fungal and oomycete hyphae and is the most widely distributed unidirectional growth process on the planet. It ensures spatial colonization, nutrient predation, fertilization, and symbiosis with growth speeds of up to 800 μm h-1. Although turgor-driven growth is intuitively conceivable, a closer examination of the physical processes at work in tip growth raises a paradox: growth occurs where biophysical forces are low, because of the increase in curvature in the tip. All tip-growing cells studied so far rely on the modulation of cell wall extensibility via the polarized excretion of cell wall-loosening compounds at the tip. Here, we used a series of quantitative measurements at the cellular level and a biophysical simulation approach to show that the brown alga Ectocarpus has an original tip-growth mechanism. In this alga, the establishment of a steep gradient in cell wall thickness can compensate for the variation in tip curvature, thereby modulating wall stress within the tip cell. Bootstrap analyses support the robustness of the process, and experiments with fluorescence recovery after photobleaching (FRAP) confirmed the active vesicle trafficking in the shanks of the apical cell, as inferred from the model. In response to auxin, biophysical measurements change in agreement with the model. Although we cannot strictly exclude the involvement of a gradient in mechanical properties in Ectocarpus morphogenesis, the viscoplastic model of cell wall mechanics strongly suggests that brown algae have evolved an alternative strategy of tip growth. This strategy is largely based on the control of cell wall thickness rather than fluctuations in cell wall mechanical properties.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Heath IB. Tip growth in plant and fungal cells Harcourt Brace Jovanovich. San Diego: Academic Press; 1990.
-
- Benkert R, Obermeyer G, Bentrup F-W. The turgor pressure of growing lily pollen tubes. Protoplasma. 1997;198: 1–8. 10.1007/BF01282125 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

