Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems
- PMID: 30640940
- PMCID: PMC6331146
- DOI: 10.1371/journal.pone.0210728
Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems
Abstract
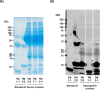
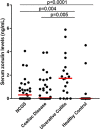
The protein, zonulin, has emerged as a popular serological marker to assess the integrity of the intestinal mucosal barrier. However, there is limited information on the utility of serum zonulin to indicate gastrointestinal disease and the validity of zonulin detection in widely-used commercial assays. The current study reports differences in zonulin levels across patient groups with gastrointestinal dysfunction compared with healthy individuals, though methodological inconsistencies indicated that actual zonulin protein was not detected by the commercial assays applied. The nature of the assays' detected antigen was investigated using immunoprecipitation followed by mass spectrometric analysis and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by protein staining. Top matches of the assays' detected antigen included haptoglobin and complement C3 for the assay manufactured by CUSABIO (Wuhan, China) and complement C3 for the assay manufactured by Immundiagnostik AG (Bensheim, Germany). These findings confirm that current commercial zonulin assays are not detecting the actual protein as prehaptoglobin-2. Until assay methodology is improved, we advise the greater scientific and medical community to exercise caution in considering the measurement of serum zonulin as a marker of mucosal barrier integrity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(39):16799–804. 10.1073/pnas.0906773106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

