Programmable Molecular Scissors: Applications of a New Tool for Genome Editing in Biotech
- PMID: 30641475
- PMCID: PMC6330515
- DOI: 10.1016/j.omtn.2018.11.016
Programmable Molecular Scissors: Applications of a New Tool for Genome Editing in Biotech
Abstract
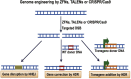
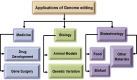
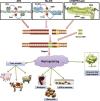
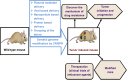
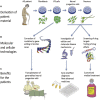
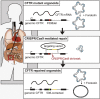
Targeted genome editing is an advanced technique that enables precise modification of the nucleic acid sequences in a genome. Genome editing is typically performed using tools, such as molecular scissors, to cut a defined location in a specific gene. Genome editing has impacted various fields of biotechnology, such as agriculture; biopharmaceutical production; studies on the structure, regulation, and function of the genome; and the creation of transgenic organisms and cell lines. Although genome editing is used frequently, it has several limitations. Here, we provide an overview of well-studied genome-editing nucleases, including single-stranded oligodeoxynucleotides (ssODNs), transcription activator-like effector nucleases (TALENs), zinc-finger nucleases (ZFNs), and CRISPR-Cas9 RNA-guided nucleases (CRISPR-Cas9). To this end, we describe the progress toward editable nuclease-based therapies and discuss the minimization of off-target mutagenesis. Future prospects of this challenging scientific field are also discussed.
Keywords: CRISPR-Cas9; DSB; HDR; NHEJ; TALENs; ZFNs; genome editing; nucleases; off-target mutagenesis; ssODNs.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Krueger U., Bergauer T., Kaufmann B., Wolter I., Pilk S., Heider-Fabian M., Kirch S., Artz-Oppitz C., Isselhorst M., Konrad J. Insights into effective RNAi gained from large-scale siRNA validation screening. Oligonucleotides. 2007;17:237–250. - PubMed
-
- McManus M.T., Sharp P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002;3:737–747. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

