Bone Metastasis Pain, from the Bench to the Bedside
- PMID: 30641973
- PMCID: PMC6359191
- DOI: 10.3390/ijms20020280
Bone Metastasis Pain, from the Bench to the Bedside
Abstract
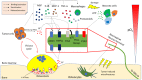
Bone is the most frequent site of metastasis of the most common cancers in men and women. Bone metastasis incidence has been steadily increasing over the years, mainly because of higher life expectancy in oncologic patients. Although bone metastases are sometimes asymptomatic, their consequences are most often devastating, impairing both life quality and expectancy, due to the occurrence of the skeletal-related events, including bone fractures, hypercalcemia and spinal cord compression. Up to 75% of patients endure crippling cancer-induced bone pain (CIBP), against which we have very few weapons. This review's purpose is to discuss the molecular and cellular mechanisms that lead to CIBP, including how cancer cells convert the bone "virtuous cycle" into a cancer-fuelling "vicious cycle", and how this leads to the release of molecular mediators of pain, including protons, neurotrophins, interleukins, chemokines and ATP. Preclinical tests and assays to evaluate CIBP, including the incapacitance tester (in vivo), and neuron/glial activation in the dorsal root ganglia/spinal cord (ex vivo) will also be presented. Furthermore, current therapeutic options for CIBP are quite limited and nonspecific and they will also be discussed, along with up-and-coming options that may render CIBP easier to treat and let patients forget they are patients.
Keywords: bone metastasis; bone pain; osteoblasts; osteoclasts; skeletal-related events.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

