Homeostasis of Glucose and Lipid in Non-Alcoholic Fatty Liver Disease
- PMID: 30642126
- PMCID: PMC6359196
- DOI: 10.3390/ijms20020298
Homeostasis of Glucose and Lipid in Non-Alcoholic Fatty Liver Disease
Abstract
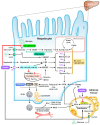
Industrialized society-caused dysregular human behaviors and activities such as overworking, excessive dietary intake, and sleep deprivation lead to perturbations in the metabolism and the development of metabolic syndrome. Non-alcoholic fatty liver disease (NAFLD), the most common chronic liver disease worldwide, affects around 30% and 25% of people in Western and Asian countries, respectively, which leads to numerous medical costs annually. Insulin resistance is the major hallmark of NAFLD and is crucial in the pathogenesis and for the progression from NAFLD to non-alcoholic steatohepatitis (NASH). Excessive dietary intake of saturated fats and carbohydrate-enriched foods contributes to both insulin resistance and NAFLD. Once NAFLD is established, insulin resistance can promote the progression to the more severe state of liver endangerment like NASH. Here, we review current and potential studies for understanding the complexity between insulin-regulated glycolytic and lipogenic homeostasis and the underlying causes of NAFLD. We discuss how disruption of the insulin signal is associated with various metabolic disorders of glucoses and lipids that constitute both the metabolic syndrome and NAFLD.
Keywords: glucose; lipid; non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

