A novel mutation in SEPN1 causing rigid spine muscular dystrophy 1: a Case report
- PMID: 30642275
- PMCID: PMC6332642
- DOI: 10.1186/s12881-018-0743-1
A novel mutation in SEPN1 causing rigid spine muscular dystrophy 1: a Case report
Abstract
Background: Muscular dystrophies are a clinically and genetically heterogeneous group of disorders characterized by variable degrees of progressive muscle degeneration and weakness. There is a wide variability in the age of onset, symptoms and rate of progression in subtypes of these disorders. Herein, we present the results of our study conducted to identify the pathogenic genetic variation involved in our patient affected by rigid spine muscular dystrophy.
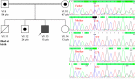
Case presentation: A 14-year-old boy, product of a first-cousin marriage, was enrolled in our study with failure to thrive, fatigue, muscular dystrophy, generalized muscular atrophy, kyphoscoliosis, and flexion contracture of the knees and elbows. Whole-exome sequencing (WES) was carried out on the DNA of the patient to investigate all coding regions and uncovered a novel, homozygous missense mutation in SEPN1 gene (c. 1379 C > T, p.Ser460Phe). This mutation has not been reported before in different public variant databases and also our database (BayanGene), so it is classified as a variation of unknown significance (VUS). Subsequently, it was confirmed that the novel variation was homozygous in our patient and heterozygous in his parents. Different bioinformatics tools showed the damaging effects of the variant on protein. Multiple sequence alignment using BLASTP on ExPASy and WebLogo, revealed the conservation of the mutated residue.
Conclusion: We reported a novel homozygous mutation in SEPN1 gene that expands our understanding of rigid spine muscular dystrophy. Although bioinformatics analyses of results were in favor of the pathogenicity of the mutation, functional studies are needed to establish the pathogenicity of the variant.
Keywords: Muscular dystrophies; Novel mutation; Rigid spine muscular dystrophy; SEPN1; Selenoproteins.
Conflict of interest statement
Ethics approval and consent to participate
Ethic committee at Persian BayanGene Research and Training Center, Dr. Faghihi’s Medical Genetic Center has approved the study and parents of the affected individual have signed written informed consent indicating their voluntary contribution to the current study. A copy of the consent is available for review by the Editor of this journal.
Consent for publication
Both patient’s legal guardians (parents) have signed informed consent to participate in this study and both families consented to publish result of study, including medical data and images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Targeted next generation sequencing identifies two novel mutations in SEPN1 in rigid spine muscular dystrophy 1.Oncotarget. 2016 Dec 20;7(51):83843-83849. doi: 10.18632/oncotarget.13337. Oncotarget. 2016. PMID: 27863379 Free PMC article.
-
SEPN1-related Rigid Spine Muscular Dystrophy.Indian J Pediatr. 2018 Nov;85(11):1033-1034. doi: 10.1007/s12098-018-2713-1. Epub 2018 May 31. Indian J Pediatr. 2018. PMID: 29850975 No abstract available.
-
The first report of two homozygous sequence variants in FKRP and SELENON genes associated with syndromic congenital muscular dystrophy in Iran: Further expansion of the clinical phenotypes.J Gene Med. 2020 Dec;22(12):e3265. doi: 10.1002/jgm.3265. Epub 2020 Sep 29. J Gene Med. 2020. PMID: 32864802
-
Reducing body myopathy and other FHL1-related muscular disorders.Semin Pediatr Neurol. 2011 Dec;18(4):257-63. doi: 10.1016/j.spen.2011.10.007. Semin Pediatr Neurol. 2011. PMID: 22172421 Review.
-
Genetics and muscle pathology in the diagnosis of muscular dystrophies: An update.Indian J Pathol Microbiol. 2022 May;65(Supplement):S259-S270. doi: 10.4103/ijpm.ijpm_1074_21. Indian J Pathol Microbiol. 2022. PMID: 35562158 Review.
Cited by
-
SELENON-Related Myopathy Across the Life Span, a Cross-Sectional Study for Preparing Trial Readiness.J Neuromuscul Dis. 2023;10(6):1055-1074. doi: 10.3233/JND-221673. J Neuromuscul Dis. 2023. PMID: 37807786 Free PMC article.
-
Genetic Disorders Associated with Metal Metabolism.Cells. 2019 Dec 9;8(12):1598. doi: 10.3390/cells8121598. Cells. 2019. PMID: 31835360 Free PMC article. Review.
-
Delayed Respiratory Insufficiency and Extramuscular Abnormalities in Selenoprotein N-Related Myopathies.Front Neurol. 2021 Nov 19;12:766942. doi: 10.3389/fneur.2021.766942. eCollection 2021. Front Neurol. 2021. PMID: 34867752 Free PMC article.
-
SEPN1-Related Myopathy: The Importance of Diagnosis and Challenges to Management of CMD in Resource Poor Settings.Ann Indian Acad Neurol. 2021 Nov-Dec;24(6):955-956. doi: 10.4103/aian.AIAN_655_20. Epub 2021 Mar 5. Ann Indian Acad Neurol. 2021. PMID: 35359550 Free PMC article. No abstract available.
-
A Holistic Approach to Implementing Artificial Intelligence in Lung Cancer.Indian J Surg Oncol. 2025 Feb;16(1):257-278. doi: 10.1007/s13193-024-02079-6. Epub 2024 Sep 5. Indian J Surg Oncol. 2025. PMID: 40114896 Review.

