Phase I/II trial of Durvalumab plus Tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma
- PMID: 30642290
- PMCID: PMC6332607
- DOI: 10.1186/s12885-019-5266-4
Phase I/II trial of Durvalumab plus Tremelimumab and stereotactic body radiotherapy for metastatic head and neck carcinoma
Abstract
Background: The efficacy of immunotherapy targeting the PD-1/PD-L1 pathway has previously been demonstrated in metastatic head and neck squamous cell carcinoma (HNSCC). Stereotactic Body Radiotherapy (SBRT) aims at ablating metastatic lesions and may play a synergistic role with immunotherapy. The purpose of this study is to assess the safety and efficacy of triple treatment combination (TTC) consisting of the administration of durvalumab and tremelimumab in combination with SBRT in metastatic HNSCC.
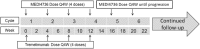
Method: This is a phase I/II single arm study that will include 35 patients with 2-10 extracranial metastatic lesions. Patients will receive durvalumab (1500 mg IV every 4 weeks (Q4W)) and tremelimumab (75 mg IV Q4W for a total of 4 doses) until progression, unacceptable toxicity or patient withdrawal. SBRT to 2-5 metastases will be administered between cycles 2 and 3 of immunotherapy. The safety of the treatment combination will be evaluated through assessment of TTC-related toxicities, defined as grade 3-5 toxicities based on Common Terminology Criteria for Adverse Events (v 4.03), occurring within 6 weeks from SBRT start, and that are definitely, probably or possibly related to the combination of all treatments. We hypothesize that dual targeting of PD-L1 and CTLA-4 pathways combined with SBRT will lead to < 35% grade 3-5 acute toxicities related to TTC. Progression free survival (PFS) will be the primary endpoint of the phase II portion of this study and will be assessed with radiological exams every 8 weeks using the RECIST version 1.1 criteria.
Discussion: The combination of synergistic dual checkpoints inhibition along with ablative radiation may significantly potentiate the local and systemic disease control. This study constitutes the first clinical trial combining effects of SBRT with dual checkpoint blockade with durvalumab and tremelimumab in the treatment of metastatic HNSCC. If positive, this study would lead to a phase III trial testing this treatment combination against standard of care in metastatic HNSCC.
Trial registration: NCT03283605 . Registration date: September 14, 2017; version 1.
Keywords: Durvalumab; Head and neck cancer; Immunotherapy; Metastatic; SBRT; Tremelimumab.
Conflict of interest statement
Ethics approval and consent to participate
The proposed study has been approved by the Centre Hospitalier de l’Université de Montreal IRB.
Consent for publication
Not applicable.
Competing interests
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials