Interplay between Influenza Virus and the Host RNA Polymerase II Transcriptional Machinery
- PMID: 30642766
- PMCID: PMC6467242
- DOI: 10.1016/j.tim.2018.12.013
Interplay between Influenza Virus and the Host RNA Polymerase II Transcriptional Machinery
Abstract
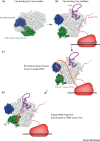
The influenza virus RNA-dependent RNA polymerase (RdRP) cleaves the 5' end of nascent capped host RNAs and uses the capped RNA fragment to prime viral transcription in a mechanism called 'cap snatching'. Cap snatching requires an intimate association between influenza RdRP and cellular RNA polymerase II (Pol II), which is the source of nascent capped host RNAs targeted by influenza virus. Recent structural studies have revealed how influenza RdRP binds to Pol II and how this binding promotes the initiation of viral transcription by influenza RdRP. In this review we focus on these recent insights into the mechanism of cap snatching by influenza virus and the impact of cap snatching on host gene expression during influenza virus infection.
Keywords: CTD; Pol II; RNA polymerase; cap snatching; influenza virus.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

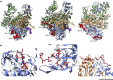

References
-
- Krammer F., Palese P. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 2015;14:167–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

