Adverse events associated with immune checkpoint inhibitor treatment for cancer
- PMID: 30642824
- PMCID: PMC6333545
- DOI: 10.1503/cmaj.180870
Adverse events associated with immune checkpoint inhibitor treatment for cancer
Conflict of interest statement
Competing interests: Marie Hudson reports receiving a grant from Bristol-Myers Squibb, outside the submitted work. Khashayar Esfahani reports receiving speaker’s fees from Bristol-Myers Squibb, outside the submitted work. Wilson Miller reports receiving grants from Bristol-Myers Squibb, Merck, Roche, Novartis, AstraZeneca, Amgen, Bayer, MedImmune and GlaxoSmithKline, outside the submitted work, as well as personal fees from Bristol-Myers Squibb, Merck, Roche, Novartis, Amgen and GlaxoSmithKline. No other competing interests were declared.
Figures
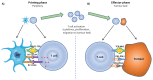
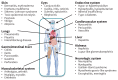

References
-
- Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol 2018;36:1675–84. - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
