Isoniazid Bactericidal Activity Involves Electron Transport Chain Perturbation
- PMID: 30642937
- PMCID: PMC6395907
- DOI: 10.1128/AAC.01841-18
Isoniazid Bactericidal Activity Involves Electron Transport Chain Perturbation
Abstract
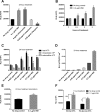
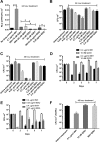
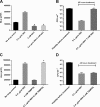
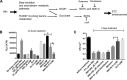
Accumulating evidence suggests that the bactericidal activity of some antibiotics may not be directly initiated by target inhibition. The activity of isoniazid (INH), a key first-line bactericidal antituberculosis drug currently known to inhibit mycolic acid synthesis, becomes extremely poor under stress conditions, such as hypoxia and starvation. This suggests that the target inhibition may not fully explain the bactericidal activity of the drug. Here, we report that INH rapidly increased Mycobacterium bovis BCG cellular ATP levels and enhanced oxygen consumption. The INH-triggered ATP increase and bactericidal activity were strongly compromised by Q203 and bedaquiline, which inhibit mycobacterial cytochrome bc1 and FoF1 ATP synthase, respectively. Moreover, the antioxidant N-acetylcysteine (NAC) but not 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) abrogated the INH-triggered ATP increase and killing. These results reveal a link between the energetic (ATP) perturbation and INH's killing. Furthermore, the INH-induced energetic perturbation and killing were also abrogated by chemical inhibition of NADH dehydrogenases (NDHs) and succinate dehydrogenases (SDHs), linking INH's bactericidal activity further to the electron transport chain (ETC) perturbation. This notion was also supported by the observation that INH dissipated mycobacterial membrane potential. Importantly, inhibition of cytochrome bd oxidase significantly reduced cell recovery during INH challenge in a culture settling model, suggesting that the respiratory reprogramming to the cytochrome bd oxidase contributes to the escape of INH killing. This study implicates mycobacterial ETC perturbation through NDHs, SDHs, cytochrome bc1, and FoF1 ATP synthase in INH's bactericidal activity and pinpoints the participation of the cytochrome bd oxidase in protection against this drug under stress conditions.
Keywords: Mycobacterium tuberculosis; Q203; bedaquiline; electron transport chain; isoniazid; persistence.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- WHO. 2017. Global tuberculosis report 2017. WHO, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

