Bacterial killing by complement requires membrane attack complex formation via surface-bound C5 convertases
- PMID: 30643019
- PMCID: PMC6376327
- DOI: 10.15252/embj.201899852
Bacterial killing by complement requires membrane attack complex formation via surface-bound C5 convertases
Abstract
The immune system kills bacteria by the formation of lytic membrane attack complexes (MACs), triggered when complement enzymes cleave C5. At present, it is not understood how the MAC perturbs the composite cell envelope of Gram-negative bacteria. Here, we show that the role of C5 convertase enzymes in MAC assembly extends beyond the cleavage of C5 into the MAC precursor C5b. Although purified MAC complexes generated from preassembled C5b6 perforate artificial lipid membranes and mammalian cells, these components lack bactericidal activity. In order to permeabilize both the bacterial outer and inner membrane and thus kill a bacterium, MACs need to be assembled locally by the C5 convertase enzymes. Our data indicate that C5b6 rapidly loses the capacity to form bactericidal pores; therefore, bacterial killing requires both in situ conversion of C5 and immediate insertion of C5b67 into the membrane. Using flow cytometry and atomic force microscopy, we show that local assembly of C5b6 at the bacterial surface is required for the efficient insertion of MAC pores into bacterial membranes. These studies provide basic molecular insights into MAC assembly and bacterial killing by the immune system.
Keywords: Gram‐negative bacteria; atomic force microscopy; complement; convertase; membrane attack complex.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
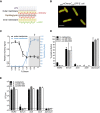
- A
Schematic representation of engineered perimCherry/cytoGFP E. coli cells that express mCherry in the periplasmic space (between the outer and inner membrane) and GFP in the cytosol.
- B
Structured illumination microscopy image of perimCherry/cytoGFP E. coli confirming localization of mCherry (red) in the periplasm and GFP (green) in the cytosol. Scale bar = 3 μm.
- C
Outer membrane damage (mCherry intensity) and inner membrane damage (% Sytox positive) of perimCherry/cytoGFP E. coli bacteria exposed to (different concentrations of) human serum. Inner membrane damage correlates with killing (samples where bacteria are killed are indicated with gray shadings and a cross, see CFU data in Fig EV1B).
- D, E
(D) Serum‐induced inner membrane damage (% Sytox positive) and (E) killing (CFU/ml) of different Gram‐negative strains depends on MAC components C5 and C8, but not on lysozyme (10% serum). Dotted line represents the detection limit of the assay.

Representative flow cytometry plots of perimCherry/cytoGFP E. coli after 30 min of exposure to buffer or 10% human serum.
Bacterial viability (via colony enumeration on agar plates) of perimCherry/cytoGFP E. coli exposed to a concentration range of serum (samples identical to Fig 1C).
Successful depletion of serum from lysozyme (lysozyme‐specific ELISA; black line), but sustained complement activity (CH50; red).
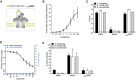
Purified MAC (denoted as C5b6MAC) can be formed by mixing preassembled C5b6 complexes with C7, C8, and C9.
Lysis of human erythrocytes after exposure to a concentration range of preassembled C5b6 in the presence of 100 nM C7. After washing, erythrocytes were exposed to 20 nM C8 and 100 nM C9 for 30 min after which the OD405 nm of the supernatant was measured.
Bacterial viability of three Gram‐negative strains after exposure to buffer, 10% human serum or C5b6MAC. Buffer and serum conditions are the same as Fig 1E.
Permeabilization of the outer, but not inner membrane of perimCherry/cytoGFP E. coli cells exposed to C5b6MAC (different concentrations of C5b6 with fixed concentrations of C7‐C9).
Inner membrane damage of three Gram‐negative strains exposed to buffer, 10% serum or C5b6MAC. Buffer and serum conditions are the same as Fig 1D.

Lysis of liposomes after exposure to preassembled C5b6 with or without C7, C8, and C9. Calcein release from liposomes was determined by measuring absorbance at OD340 nm; 0.5% Triton X‐100 was used as a positive control.
Percentage lysis of rabbit erythrocytes exposed to buffer or C5b6MAC, compared to Milli‐Q (MQ) water as control (set at 100% lysis).
Killing of E. coli MG1655 after exposure to preassembled C5b6 with or without C7, C8, and C9 (at concentrations similar to (B) or at concentrations exceeding those of 100% serum (highlighted by an arrow); ± 100 nM C5b6, 600 nM C7, 350 nM C8, 900 nM C9).

Schematic overview for Conv‐MAC formation. Bacteria were labeled with C5 convertases by pre‐incubation with C5‐deficient serum (Fig EV3). Following a washing step (@), convertase‐labeled bacteria were incubated with uncleaved C5, C6, C7, C8, and C9 (termed “Conv‐MAC”).
Bacterial viability of convertase‐labeled bacterial strains exposed to buffer (Conv) or C5‐C9 (Conv‐MAC).
Bacterial viability of convertase‐labeled E. coli MG1655 exposed to a concentration range of C5 in the presence of 100 nM C6, 100 nM C7, 20 nM C8, and 100 nM C9. “Ctrl” indicates bacteria that are pretreated with heat‐inactivated ΔC5 serum. Dotted line represents the detection limit of the assay.
Bacterial viability of convertase‐labeled E. coli MG1655 exposed to C5‐C9 or conditions lacking one MAC component. As an extra control, convertase formation was blocked during ΔC5 serum incubation by adding 5 μM compstatin.
Bacterial viability of E. coli MG1655 exposed to FB depleted serum in the presence of 20 μg/ml OmCI (to deposit C4b and C3b without Bb). After washing, bacteria were exposed to C5‐C9 in the presence or absence of C1 and C2 (to generate classical pathway C5 convertases, C4b2aC3b).

- A
Schematic overview of complement activation and MAC formation on the bacterial membrane. Different recognition pathways (classical, lectin, and alternative) generate C3 convertases (C4b2a in the classical/lectin pathway, C3bBb in the alternative pathway) on the target cell surface that cleave the major complement protein C3 into C3b. C3b covalently attaches to the cell surface via a reactive thioester. At high C3b densities, C3 convertases associate with deposited C3b to form C5 convertases (C4b2aC3b in the classical/lectin pathway, C3bBbC3b in the alternative pathway). The C5 convertase then catalyzes conversion of C5 into C5a and C5b. C5b triggers MAC formation by sequential binding to C6, C7, C8, and multiple copies of C9.
- B
Incubation of E. coli MG1655 with a concentration range of C5‐depleted serum (ΔC5 serum) results in surface labeling with alternative pathway convertases (C3bBbC3b, evidence by flow cytometry analysis of surface‐bound C3b and Bb).
- C, D
Successful labeling of E. coli with C5 convertases. (C) E. coli MG1655 was pre‐incubated with 10% ΔC5 serum (labeled as: C5 convertase), heat‐inactivated ΔC5 serum (Ctrl), or ΔC5 serum supplemented with 5 μM compstatin (Ctrl). After washing, C3b deposition was measured by flow cytometry. (D) Bacteria were labeled as described in (C) and (after washing) incubated with a concentration range of C5. Release of C5a into supernatants was measured by a calcium flux‐based assay (Bestebroer et al, 2010).

Outer membrane damage (mCherry intensity) and inner membrane damage (% Sytox positive) of convertase‐labeled perimCherry/cytoGFP E. coli cells incubated with a concentration range of C5 and fixed concentrations of C6‐C9.
Inner membrane damage of perimCherry/cytoGFP E. coli exposed to a concentration range of ΔC5 serum and, after washing, to C5‐C9. As controls, bacteria were incubated with heat‐inactivated ΔC5 serum or 5 μM compstatin was added to the ΔC5 serum to block C3b deposition.
Inner membrane damage of three different convertase‐labeled bacteria exposed to buffer (Conv) or C5‐C9 (Conv‐MAC).
Confocal microscopy images of convertase‐labeled perimCherry/cytoGFP E. coli exposed to buffer (Conv) or C5‐C9 (Conv‐MAC). Unlabeled bacteria exposed to 1% serum served as control. Green = GFP, red = To‐pro‐3 DNA dye. Scale bars = 3 μm.

- A
Schematic overview of MAC assembly on convertase‐labeled bacteria by C5b6 that is locally generated by incubation with C5 and C6 (top) or by preassembled C5b6 (bottom).
- B
Bacterial viability of convertase‐labeled E. coli MG1655 exposed to Buffer (Conv), preassembled C5b6 (Conv + C5b6MAC) or a mixture of C5 and C6 (Conv‐MAC), in the presence of C7, C8, and C9. Dotted line represents the detection limit of the assay.
- C, D
(C) Inner membrane damage (% Sytox positive) and (D) outer membrane damage (mCherry) of convertase‐labeled perimCherry/cytoGFP E. coli exposed to a concentration range of preassembled C5b6 or a mixture of C5 and C6, in the presence of 100 nM C7. After washing, bacteria were exposed to 20 nM C8 and 100 nM C9.

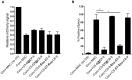
- A, B
Step‐wise assembly of MAC on convertase‐labeled bacteria. Convertase‐labeled bacteria were incubated with C5/C6 or C5/C6/C7 for 15 min, and subsequently washed (@) or treated with 10 μg/ml Eculizumab (Ecu). Then, the remaining MAC components (C7‐9 for C5/C6 or C8‐9 for C5/C6/C7, respectively) were added to the incubation mixture. In the control conditions (Conv‐MAC), the remaining MAC components were added to the incubation mixture without washing or adding an inhibitor. (A) Outer membrane damage (mCherry) and (B) inner membrane damage (% Sytox positive) were determined.
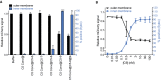
Outer and inner membrane damage of convertase‐labeled bacteria exposed to different combinations of MAC components. “@” indicates a washing step.
Outer and inner membrane damage of convertase‐labeled bacteria exposed to C5‐C8 and after washing, to a concentration range of C9.
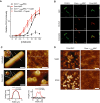
- A
Convertase‐labeled bacteria were exposed to a concentration range of either preassembled C5b6 (C5b6MAC) or a mixture of C5 and C6 (Conv‐MAC), in the presence of 100 nM C7. After washing, 20 nM C8 and 100 nM C9‐Cy3 were added. Controls at 0 nM C5b6 or C5‐C6 confirm that the detected C9‐Cy3 deposition is specifically related to MAC formation (solid lines). Proper insertion of pores was assessed by a previously described shaving method with trypsin (Moskovich & Fishelson, 2007). Bacteria were first incubated with MAC components for 30 min and subsequently treated with 20 μg/ml trypsin for 15 min at 37°C (dotted lines).
- B
Convertase‐labeled perimCherry/cytoGFP bacteria (Green) exposed to C5b6MAC or Conv‐MAC. Conditions were similar to those in (A); however, C9‐Cy5 was used to detect MAC pores (Red). 100 nM of C5 and C6 or C5b6 was used in combination with 100 nM C7, 20 nM C8, and 100 nM C9‐Cy5. Conv + C5b6MAC and Conv‐MAC images were taken in separate experiments in which laser settings were adjusted to the staining intensity of C9‐Cy5 to properly visualize pore distribution. Scale bars = 3 μm.
- C, D
Atomic force microscopy analysis of E. coli BL21 and MG1655 immobilized using the Poly‐L‐Lysine protocol. (C) Entire bacteria and high‐resolution comparisons of untreated and convertase‐labeled E. coli BL21 exposed to C5‐C9 (Conv‐MAC) for 10 min. Scale bars: 800 nm (left) and 30 nm (right). Height scales: 1 μm (left), 8 nm (top right), 22 nm (bottom right). Width of magnification boxes: 42 nm, height scales: 8 nm (top) and 13 nm (bottom). Arrows highlight E. coli porin structures; an asterisk highlights the C5b‐7 stalk. Height profiles (bottom) are shown for the white dashed lines in the images. (D) Atomic force microscopy (height and phase images) of convertase‐labeled E. coli MG1655 exposed to a mixture of C5 and C6 (Conv‐MAC) or preassembled C5b6 (Conv + C5b6MAC), in the presence of C7, C8, C9, FB, and FD. Images were generated in the same experiment. Scale bars: 50 nm. Height scales: 15 nm. This figure and three other replicates are included in Fig EV5B and C.
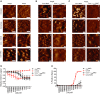
- A
Atomic force microscopy height (left) and phase (right) images of E. coli MG1655 incubated with buffer (untreated), convertases, and convertases plus either C5‐C8 (Conv + C5‐C8) or C5‐C9 (Conv‐MAC). Scale bars: 50 nm. Height scales: 5 nm (untreated), 9 nm (convertase), and 6 nm (conv + C5‐C8/MAC).
- B, C
Atomic force microscopy height (B) and phase (C) images of convertase‐labeled E. coli MG1655 exposed to either C5 and C6 or preassembled C5b6 (Conv‐MAC versus Conv + C5b6MAC) in the presence of C7‐C9, FB, and FD. Data shown correspond to four separate experiments; in each experiment, Conv‐MAC and Conv + C5b6MAC were compared directly. The upper images of (B and C) are also presented in Fig 8D. Scale bars: 50 nm. Height scales: 15 nm.
- D, E
(D) Outer membrane damage (mCherry) and (E) inner membrane damage (% Sytox positive) of non‐opsonized or convertase‐labeled bacteria incubated with 10 nM of C5 and C6 or C5b6 in the presence of 10 nM C7. After washing, a concentration range of C8 and 100 nM C9 was added. Data represent mean ± SD of 3 independent experiments.

Hypothetical model for C5 cleavage by the alternative pathway C5 convertase. The AP C5 convertase is a multimeric complex between a dimeric C3 convertase enzyme (comprised of surface‐bound non‐catalytic C3b in complex with protease Bb), together with additional surface‐bound C3b molecules (not depicted here), which are required to strengthen the affinity for C5. Hypothetical model of C3bBb (surface representation, C3b in gray, Bb in orange) bound to substrate C5 (light green, C5d domain in dark green). C3bBb is derived from the dimeric C3bBb‐SCIN complex (PDB 2WIN; Rooijakkers et al, 2009), and C5 is modeled based on superposition of the CVF‐C5 complex (PDB 3PVM; Laursen et al, 2011) on the C3b molecule from C3bBb. The right panel shows C3bBb bound to C5b (light green, C5d in dark green). The structure of C5b is derived from the structure of the C5b6 complex (PDB 4A5W; Hadders et al, 2012) and superimposed on C5 from the model in the left panel.
Superposition of C5b with the C5d domain in the pC5b6 (dark green) and C3b‐like (light blue) orientation. The C3b‐like conformation of C5d was generated based on superposition of the C5d structure (extracted from the pC5b6 structure, PDB 4A5W) on the C3d domain of the second C3b subunit from the dimeric C3bBb‐SCIN structure (PDB 2WIN).
Hypothetical structural models for C5b6 assembly by convertases. (I) Model of pC5b6 bound to C3bBb, as in (A, right). (II) Model of pC5b6, with C5d‐C6 superimposed on C5d in the C3b‐like orientation, as in (B). Note that this orientation allows C6 to extend further toward the membrane relative to the convertase. (III) Model in which C5b6 has dissociated from C3bBb, but adopted the orientation shown in (II). All structural models and superpositions were generated using UCSF Chimera (Pettersen et al, 2004).
References
-
- Al Salihi A, Ripoche J, Fontaine M (1988) Localization of a hydrophobic domain in human C5. Mol Immunol 25: 367–377 - PubMed
-
- Berends ETM, Dekkers JF, Nijland R, Kuipers A, Soppe JA, van Strijp JAG, Rooijakkers SHM (2013) Distinct localization of the complement C5b‐9 complex on gram‐positive bacteria. Cell Microbiol 15: 1955–1968 - PubMed
-
- Berends ETM, Kuipers A, Ravesloot MM, Urbanus RT, Rooijakkers SHM (2014) Bacteria under stress by complement and coagulation. FEMS Microbiol Rev 38: 1146–1171 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

