Region-specific inhibition of 14-3-3 proteins induces psychomotor behaviors in mice
- PMID: 30643138
- PMCID: PMC6386769
- DOI: 10.1038/s41537-018-0069-1
Region-specific inhibition of 14-3-3 proteins induces psychomotor behaviors in mice
Abstract
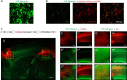
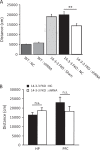
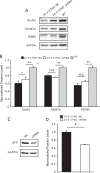
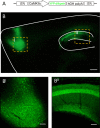
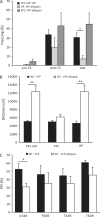
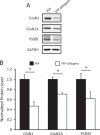
The 14-3-3 family of proteins is genetically linked to several psychiatric disorders, including schizophrenia. Our 14-3-3 functional knockout (FKO) mice, as well as other 14-3-3 knockout models, have been shown to exhibit behavioral endophenotypes related to schizophrenia. While specific forebrain regions, such as the prefrontal cortex (PFC) and hippocampus (HP), have been implicated in schizophrenic pathophysiology, the role of these brain regions in the top-down control of specific schizophrenia-associated behaviors has not been examined. Here, we used an adeno-associated virus (AAV) delivered shRNA to knock down the expression of the 14-3-3-inhibitor transgene, thus selectively restoring the function of 14-3-3 in the forebrain of the 14-3-3 FKO mice, we found that injection of the AAV-shRNA into both the PFC and the HP is necessary to attenuate psychomotor activity of the 14-3-3 FKO mice. Furthermore, we found that acute inhibition of 14-3-3, through the delivery of an AAV expressing the 14-3-3 inhibitor to both the PFC and HP, can trigger psychomotor agitation. Interestingly, when assessing the two brain regions separately, we determined that AAV-mediated expression of the 14-3-3 inhibitor specifically within the HP alone is sufficient to induce several behavioral deficits including hyperactivity, impaired associative learning and memory, and reduced sensorimotor gating. In addition, we show that post-synaptic NMDA receptor levels are regulated by acute 14-3-3 manipulations. Taken together, findings from this study directly link 14-3-3 inhibition in specific forebrain regions to certain schizophrenia-associated endophenotypes.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

