Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis
- PMID: 30643142
- PMCID: PMC6331599
- DOI: 10.1038/s41467-018-08097-7
Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis
Abstract
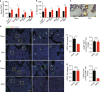
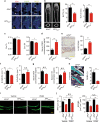
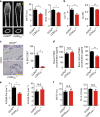
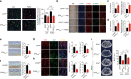
Whether sensory nerve can sense bone density or metabolic activity to control bone homeostasis is unknown. Here we found prostaglandin E2 (PGE2) secreted by osteoblastic cells activates PGE2 receptor 4 (EP4) in sensory nerves to regulate bone formation by inhibiting sympathetic activity through the central nervous system. PGE2 secreted by osteoblasts increases when bone density decreases as demonstrated in osteoporotic animal models. Ablation of sensory nerves erodes the skeletal integrity. Specifically, knockout of the EP4 gene in the sensory nerves or cyclooxygenase-2 (COX2) in the osteoblastic cells significantly reduces bone volume in adult mice. Sympathetic tone is increased in sensory denervation models, and propranolol, a β2-adrenergic antagonist, rescues bone loss. Furthermore, injection of SW033291, a small molecule to increase PGE2 level locally, significantly boostes bone formation, whereas the effect is obstructed in EP4 knockout mice. Thus, we show that PGE2 mediates sensory nerve to control bone homeostasis and promote regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Sensing bone mass.Nat Rev Rheumatol. 2019 Mar;15(3):128. doi: 10.1038/s41584-019-0181-2. Nat Rev Rheumatol. 2019. PMID: 30728449 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

