Biomimetic 3D-printed scaffolds for spinal cord injury repair
- PMID: 30643285
- PMCID: PMC6559945
- DOI: 10.1038/s41591-018-0296-z
Biomimetic 3D-printed scaffolds for spinal cord injury repair
Abstract
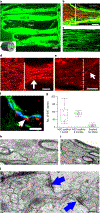
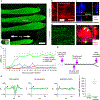
Current methods for bioprinting functional tissue lack appropriate biofabrication techniques to build complex 3D microarchitectures essential for guiding cell growth and promoting tissue maturation1. 3D printing of central nervous system (CNS) structures has not been accomplished, possibly owing to the complexity of CNS architecture. Here, we report the use of a microscale continuous projection printing method (μCPP) to create a complex CNS structure for regenerative medicine applications in the spinal cord. μCPP can print 3D biomimetic hydrogel scaffolds tailored to the dimensions of the rodent spinal cord in 1.6 s and is scalable to human spinal cord sizes and lesion geometries. We tested the ability of µCPP 3D-printed scaffolds loaded with neural progenitor cells (NPCs) to support axon regeneration and form new 'neural relays' across sites of complete spinal cord injury in vivo in rodents1,2. We find that injured host axons regenerate into 3D biomimetic scaffolds and synapse onto NPCs implanted into the device and that implanted NPCs in turn extend axons out of the scaffold and into the host spinal cord below the injury to restore synaptic transmission and significantly improve functional outcomes. Thus, 3D biomimetic scaffolds offer a means of enhancing CNS regeneration through precision medicine.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


Comment in
-
Tele-ophthalmology for diabetic retinopathy screening: 8 years of experience.Arch Soc Esp Oftalmol. 2017 Feb;92(2):63-70. doi: 10.1016/j.oftal.2016.08.006. Epub 2016 Oct 15. Arch Soc Esp Oftalmol. 2017. PMID: 27756515 English, Spanish.
-
3-Dimensional Printing a Novel Framework for Spinal Cord Injury Repair.Neurosurgery. 2019 Aug 1;85(2):E192-E193. doi: 10.1093/neuros/nyz169. Neurosurgery. 2019. PMID: 31304549 No abstract available.
References
-
- NSCISC Annual Statistical Report - Model Systems Public Version (National Spinal Cord Injury Statistical Center, University of Alabama at Birmingham, 2014).
-
- Murphy SV & Atala A 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

