Ion Therapy: A Novel Strategy for Acute Myocardial Infarction
- PMID: 30643722
- PMCID: PMC6325593
- DOI: 10.1002/advs.201801260
Ion Therapy: A Novel Strategy for Acute Myocardial Infarction
Erratum in
-
Erratum: Ion Therapy: A Novel Strategy for Acute Myocardial Infarction.Adv Sci (Weinh). 2020 May 20;7(10):2000544. doi: 10.1002/advs.202000544. eCollection 2020 May. Adv Sci (Weinh). 2020. PMID: 32440490 Free PMC article.
Abstract
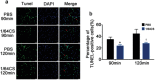
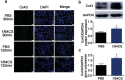
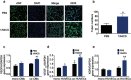
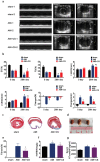
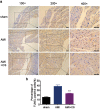
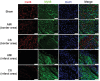
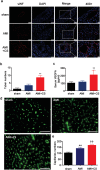
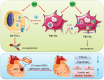
Although numerous therapies are widely applied clinically and stem cells and/or biomaterial based in situ implantations have achieved some effects, few of these have observed robust myocardial regeneration. The beneficial effects on cardiac function and structure are largely acting through paracrine signaling, which preserve the border-zone around the infarction, reduce apoptosis, blunt adverse remodeling, and promote angiogenesis. Ionic extracts from biomaterials have been proven to stimulate paracrine effects and promote cell-cell communications. Here, the paracrine stimulatory function of bioactive ions derived from biomaterials is integrated into the clinical concept of administration and proposed "ion therapy" as a novel strategy for myocardial infarction. In vitro, silicon- enriched ion extracts significantly increase cardiomyocyte viability and promote cell-cell communications, thus stimulating vascular formation via a paracrine effect under glucose/oxygen deprived conditions. In vivo, by intravenous injection, the bioactive silicon ions act as "diplomats" and promote crosstalk in myocardial cells, stimulate angiogenesis, and improve cardiac function post-myocardial infarction.
Keywords: acute myocardial infarction; bioactive ions; therapy.
Figures



References
-
- Roger V. L., Go A. S., Lloyd‐Jones D. M., Adams R. J., Berry J. D., Brown T. M., Carnethon M. R., Dai S., De Simone G., Ford E. S., Fox C. S., Fullerton H. J., Gillespie C., Greenlund K. J., Hailpern S. M., Heit J. A., Ho P. M., Howard V. J., Kissela B. M., Kittner S. J., Lackland D. T., Lichtman J. H., Lisabeth L. D., Makuc D. M., Marcus G. M., Marelli A., Matchar D. B., Mcdermott M. M., Meigs J. B., Moy C. S., Mozaffarian D., Mussolino M. E., Nichol G., Paynter N. P., Rosamond W. D., Sorlie P. D., Stafford R. S., Turan T. N., Turner M. B., Wong N. D., Wylie‐Rosett J., American Heart Association Statistics Committee and Stroke Statistics Subcommittee , Circulation 2011, 123, e18.
-
- Benjamin E. J., Virani S. S., Callaway C. W., Chamberlain A. M., Chang A. R., Cheng S., Chiuve S. E., Cushman M., Delling F. N., Deo R., De Ferranti S. D., Ferguson J. F., Fornage M., Gillespie C., Isasi C. R., Jimenez M. C., Jordan L. C., Judd S. E., Lackland D., Lichtman J. H., Lisabeth L., Liu S., Longenecker C. T., Lutsey P. L., Mackey J. S., Matchar D. B., Matsushita K., Mussolino M. E., Nasir K., O'flaherty M., Palaniappan L. P., Pandey A., Pandey D. K., Reeves M. J., Ritchey M. D., Rodriguez C. J., Roth G. A., Rosamond W. D., Sampson U. K. A., Satou G. M., Shah S. H., Spartano N. L., Tirschwell D. L., Tsao C. W., Voeks J. H., Willey J. Z., Wilkins J. T., Wu J. H., Alger H. M., Wong S. S., Muntner P., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee , Circulation 2018, 137, e67. - PubMed
-
- Heldman A. W., Difede D. L., Fishman J. E., Zambrano J. P., Trachtenberg B. H., Karantalis V., Mushtaq M., Williams A. R., Suncion V. Y., Mcniece I. K., Ghersin E., Soto V., Lopera G., Miki R., Willens H., Hendel R., Mitrani R., Pattany P., Feigenbaum G., Oskouei B., Byrnes J., Lowery M. H., Sierra J., Pujol M. V., Delgado C., Gonzalez P. J., Rodriguez J. E., Bagno L. L., Rouy D., Altman P., Foo C. W., Da Silva J., Anderson E., Schwarz R., Mendizabal A., Hare J. M., JAMA 2014, 311, 62. - PMC - PubMed
-
- Segers V. F., Lee R. T., Circ. Res. 2011, 109, 910. - PubMed
LinkOut - more resources
Full Text Sources
