Frozen thawed embryo transfer cycles; A comparison of pregnancy outcomes with and without prior pituitary suppression by GnRH agonists: An RCT
- PMID: 30643866
- PMCID: PMC6312713
Frozen thawed embryo transfer cycles; A comparison of pregnancy outcomes with and without prior pituitary suppression by GnRH agonists: An RCT
Abstract
Background: To perform an in-vitro fertilization cycle, pretreatment with gonadotropin-releasing hormone (GnRH) agonist is widely used as a part of controlled ovarian hyper-stimulation protocols to prevent endogenous luteinizing hormone surge and spontaneous ovulation. GnRH agonist pretreatment is relatively costly and there is a risk of hypo estrogenic side effect. It would also lengthen the preparation period until pituitary desensitization occurs.
Objective: Our study is aimed at evaluating the pregnancy outcome rate of frozen thawed embryo transfer with and without GnRH agonists pretreatment.
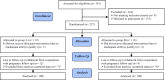
Materials and methods: Women with documented infertility who were candidate for frozen thawed embryo transfer were recruited and randomly assigned to two groups. In group A (n=100), patients received GnRH agonist, Buserelin, to induce pituitary desensitization prior to endometrial preparation and embryo transfer. Individuals in group B (n=100) received steroid manipulation without prior down-regulation of the pituitary. Chemical pregnancy, implantation rate, clinical pregnancy and ongoing pregnancy were measured and statistically compared between the two groups.
Results: None of the outcome measures including clinical and chemical pregnancy rates, implantation rate, and ongoing pregnancy rate showed significant difference between the two groups. Similarly, the rate of miscarriage did not vary between the two groups.
Conclusion: In this study, we found that removing the GnRH agonists pretreatment from the programmed cycles did not negatively influence the pregnancy outcome or implantation rate. Moreover, it will cause a considerable reduction in cost of assisted reproductive technology as well as adverse effects related to GnRH agonists, while having a favorable implantation and pregnancy outcomes.
Keywords: Agonist; Embryo transfers; Gonadotropin releasing hormone; In vitro fertilization; Ovarian stimulation.
Conflict of interest statement
Authors declare no conflict of interests.
Figures
References
-
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. - PubMed
-
- Pacchiarotti A, Selman H, Valeri C, Napoletano S, Sbracia M, Antonini G, et al. Ovarian stimulation protocol in IVF: An up-to-date review of the literature. Curr Pharm Biotechnol. 2016;17:303–315. - PubMed
-
- Bals-Pratsch M, Al-Hasani S, Schopper B, Diedrich C, Hoepfner AS, Weiss J, et al. A simple, inexpensive and effective artificial cycle with exogenous transdermal oestradiol and vaginal progesterone for the transfer of cryopreserved pronucleated human oocytes in women with normal cycles. Hum Reprod. 1999;14 :222–230. - PubMed
-
- Ben-Nun I, Shulman A. Induction of artificial endometrial cycles with s oestrogen implants and injectable progesterone in in-vitro fertilization treatment with donated oocytes: a preliminary report. Hum Reprod. 1997;12:2267–2270. - PubMed
LinkOut - more resources
Full Text Sources