Sample enrichment for clinical trials to show delay of onset in huntington disease
- PMID: 30644132
- PMCID: PMC8121118
- DOI: 10.1002/mds.27595
Sample enrichment for clinical trials to show delay of onset in huntington disease
Abstract
Background: Disease-modifying clinical trials in persons without symptoms are often limited in methods to assess the impact associated with experimental therapeutics. This study suggests sample enrichment approaches to facilitate preventive trials to delay disease onset in individuals with the dominant gene for Huntington disease.
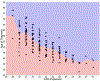
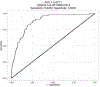
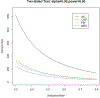
Methods: Using published onset prediction indexes, we conducted the receiver operating curve analysis for diagnosis within a 3-year clinical trial time frame. We determined optimal cut points on the indexes for participant recruitment and then conducted sample size and power calculations to detect varying effect sizes for treatment efficacy in reducing 3-year rates of disease onset (or diagnosis).
Results: Area under the curve for 3 onset prediction indexes all demonstrated excellent value in sample enrichment methodology, with the best-performing index being the multivariate risk score (MRS).
Conclusions: This study showed that conducting an intervention trial in premanifest and prodromal individuals with the gene expansion for Huntington disease is highly feasible using sample enrichment recruitment methods. Ongoing natural history studies are highly likely to indicate additional markers of disease prior to diagnosis. Statistical modeling of identified markers can facilitate participant enrichment to increase the likelihood of detecting a difference between treatment arms in a cost-effective and efficient manner. Such variations may expedite translation of emerging therapies to persons in an earlier phase of the disease.
Trial registration: PREDICT-HD is registered with www.clinicaltrials.gov, number NCT00051324. © 2019 International Parkinson and Movement Disorder Society.
Keywords: Clinical trials methodology/study design; genetics; huntington disease; movement disorders; prevention.
© 2019 International Parkinson and Movement Disorder Society.
Figures
Similar articles
-
Time to Functional Loss as an Endpoint in Huntington's Disease Trials: Enrichment and Sample Size.Mov Disord. 2024 Oct;39(10):1809-1816. doi: 10.1002/mds.29963. Epub 2024 Aug 5. Mov Disord. 2024. PMID: 39101272
-
Design optimization for clinical trials in early-stage manifest Huntington's disease.Mov Disord. 2017 Nov;32(11):1610-1619. doi: 10.1002/mds.27122. Epub 2017 Sep 14. Mov Disord. 2017. PMID: 28906031
-
Clinical and Biomarker Changes in Premanifest Huntington Disease Show Trial Feasibility: A Decade of the PREDICT-HD Study.Front Aging Neurosci. 2014 Apr 22;6:78. doi: 10.3389/fnagi.2014.00078. eCollection 2014. Front Aging Neurosci. 2014. PMID: 24795630 Free PMC article.
-
Preclinical motor manifestations of Huntington disease.Handb Clin Neurol. 2017;144:93-98. doi: 10.1016/B978-0-12-801893-4.00007-9. Handb Clin Neurol. 2017. PMID: 28947128 Review.
-
Advances in clinical trials for movement disorders.Mov Disord. 2015 Sep 15;30(11):1580-7. doi: 10.1002/mds.26371. Epub 2015 Aug 26. Mov Disord. 2015. PMID: 26307591 Review.
Cited by
-
Understanding the implications of a complete case analysis for regression models with a right-censored covariate.Am Stat. 2024;78(3):335-344. doi: 10.1080/00031305.2023.2282629. Epub 2023 Dec 21. Am Stat. 2024. PMID: 39070115 Free PMC article.
-
clusterMLD: An Efficient Hierarchical Clustering Method for Multivariate Longitudinal Data.J Comput Graph Stat. 2023;32(3):1131-1144. doi: 10.1080/10618600.2022.2149540. Epub 2023 Jan 12. J Comput Graph Stat. 2023. PMID: 37859643 Free PMC article.
-
Forecasting individual progression trajectories in Huntington disease enables more powered clinical trials.Sci Rep. 2022 Nov 7;12(1):18928. doi: 10.1038/s41598-022-18848-8. Sci Rep. 2022. PMID: 36344508 Free PMC article.
-
Tracking Huntington's Disease Progression Using Motor, Functional, Cognitive, and Imaging Markers.Mov Disord. 2021 Oct;36(10):2282-2292. doi: 10.1002/mds.28650. Epub 2021 May 20. Mov Disord. 2021. PMID: 34014005 Free PMC article.
-
HDQLIFE and neuro-QoL physical function measures: Responsiveness in persons with huntington's disease.Mov Disord. 2020 Feb;35(2):326-336. doi: 10.1002/mds.27908. Epub 2019 Nov 14. Mov Disord. 2020. PMID: 31724237 Free PMC article.
References
-
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med 2014;275(3):251–83. doi: 10.1111/joim.12191. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

