Direct Observation of Aryl Gold(I) Carbenes that Undergo Cyclopropanation, C-H Insertion, and Dimerization Reactions
- PMID: 30644625
- PMCID: PMC6593763
- DOI: 10.1002/anie.201814577
Direct Observation of Aryl Gold(I) Carbenes that Undergo Cyclopropanation, C-H Insertion, and Dimerization Reactions
Abstract
Mesityl gold(I) carbenes lacking heteroatom stabilization or shielding ancillary ligands have been generated and spectroscopically characterized from chloro(mesityl)methylgold(I) carbenoids bearing JohnPhos-type ligands by chloride abstraction with GaCl3 . The aryl carbenes react with PPh3 and alkenes to give stable phosphonium ylides and cyclopropanes, respectively. Oxidation with pyridine N-oxide and intermolecular C-H insertion to cyclohexane have also been observed. In the absence of nucleophiles, a bimolecular reaction, similar to that observed for other metal carbenes, leads to a symmetrical alkene.
Keywords: carbenes; carbenoids; density-functional calculations; gold; structure elucidation.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

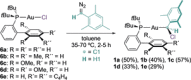





References
Grants and funding
LinkOut - more resources
Full Text Sources

