Quantifying Competition among Mitochondrial Protein Acylation Events Induced by Ethanol Metabolism
- PMID: 30644754
- PMCID: PMC6450748
- DOI: 10.1021/acs.jproteome.8b00800
Quantifying Competition among Mitochondrial Protein Acylation Events Induced by Ethanol Metabolism
Abstract
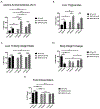
Mitochondrial dysfunction is one of many key factors in the etiology of alcoholic liver disease (ALD). Lysine acetylation is known to regulate numerous mitochondrial metabolic pathways, and recent reports demonstrate that alcohol-induced protein acylation negatively impacts these processes. To identify regulatory mechanisms attributed to alcohol-induced protein post-translational modifications, we employed a model of alcohol consumption within the context of wild type (WT), sirtuin 3 knockout (SIRT3 KO), and sirtuin 5 knockout (SIRT5 KO) mice to manipulate hepatic mitochondrial protein acylation. Mitochondrial fractions were examined by label-free quantitative HPLC-MS/MS to reveal competition between lysine acetylation and succinylation. A class of proteins defined as "differential acyl switching proteins" demonstrate select sensitivity to alcohol-induced protein acylation. A number of these proteins reveal saturated lysine-site occupancy, suggesting a significant level of differential stoichiometry in the setting of ethanol consumption. We hypothesize that ethanol downregulates numerous mitochondrial metabolic pathways through differential acyl switching proteins. Data are available via ProteomeXchange with identifier PXD012089.
Keywords: acetylation; alcoholic liver disease; mass spectrometry; mitochondria; proteomics; sirtuins; succinylation.
Conflict of interest statement
The authors declare no competing interests.
Figures



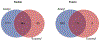





References
-
- CDC Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI) Average for United States 2006–2010 Alcohol-Attributable Deaths Due to Excessive Alcohol Use. https://www.cdc.gov/mmwr/Dreview/mmwrhtml/mm6310a2.htm: Center for Disease Control and Prevention: Atlanta, GA, 2013.
-
- NIAAA, NIAAA Alcohol Facts and Statistics https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/al....
-
- WHO Global Status Report on Alcohol and Health 2014. http://aDDS.who.int/iris/bitstream/handle/10665/112736/9789240692763_eng....; World Health Organization:Geneva, 2014.
-
- Sacks JJ; Gonzales KR; Bouchery EE; Tomedi LE; Brewer RD, 2010 National and State Costs of Excessive Alcohol Consumption. American journal of preventive medicine 2015,49 (5), e73–e79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

