Megakaryocytes as immune cells
- PMID: 30645026
- PMCID: PMC6984013
- DOI: 10.1002/JLB.MR0718-261RR
Megakaryocytes as immune cells
Abstract
Platelets play well-recognized roles in inflammation, but their cell of origin-the megakaryocyte-is not typically considered an immune lineage. Megakaryocytes are large polyploid cells most commonly identified in bone marrow. Egress via sinusoids enables migration to the pulmonary capillary bed, where elaboration of platelets can continue. Beyond receptors involved in hemostasis and thrombosis, megakaryocytes express receptors that confer immune sensing capacity, including TLRs and Fc-γ receptors. They control the proliferation of hematopoietic cells, facilitate neutrophil egress from marrow, possess the capacity to cross-present antigen, and can promote systemic inflammation through microparticles rich in IL-1. Megakaryocytes internalize other hematopoietic lineages, especially neutrophils, in an intriguing cell-in-cell interaction termed emperipolesis. Together, these observations implicate megakaryocytes as direct participants in inflammation and immunity.
Keywords: IL-1; inflammation; lung; microparticle; platelets.
©2019 Society for Leukocyte Biology.
Conflict of interest statement
Conflict of Interest Disclosure
The authors declare no relevant conflict of interest.
Figures
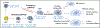



References
-
- Raslova H, Roy L, Vourc’h C, et al. Megakaryocyte polyploidization is associated with a functional gene amplification. Blood. 2003;101(2):541–544. - PubMed
-
- Hancock V, Martin JF, Lelchuk R. The relationship between human megakaryocyte nuclear DNA content and gene expression. Br J Haematol. 1993;85(4):692–697. - PubMed
-
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, et al. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81(5):695–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

