Biophysical Characterization of a Disabled Double Mutant of Soybean Lipoxygenase: The "Undoing" of Precise Substrate Positioning Relative to Metal Cofactor and an Identified Dynamical Network
- PMID: 30645119
- PMCID: PMC6353671
- DOI: 10.1021/jacs.8b10992
Biophysical Characterization of a Disabled Double Mutant of Soybean Lipoxygenase: The "Undoing" of Precise Substrate Positioning Relative to Metal Cofactor and an Identified Dynamical Network
Abstract
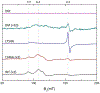
Soybean lipoxygenase (SLO) has served as a prototype for understanding the molecular origin of enzymatic rate accelerations. The double mutant (DM) L546A/L754A is considered a dramatic outlier, due to the unprecedented size and near temperature-independence of its primary kinetic isotope effect, low catalytic efficiency, and elevated enthalpy of activation. To uncover the physical basis of these features, we herein apply three structural probes: hydrogen-deuterium exchange mass spectrometry, room-temperature X-ray crystallography and EPR spectroscopy on four SLO variants (wild-type (WT) enzyme, DM, and the two parental single mutants, L546A and L754A). DM is found to incorporate features of each parent, with the perturbation at position 546 predominantly influencing thermally activated motions that connect the active site to a protein-solvent interface, while mutation at position 754 disrupts the ligand field and solvation near the cofactor iron. However, the expanded active site in DM leads to more active site water molecules and their associated hydrogen bond network, and the individual features from L546A and L754A alone cannot explain the aggregate kinetic properties for DM. Using recently published QM/MM-derived ground-state SLO-substrate complexes for WT and DM, together with the thorough structural analyses presented herein, we propose that the impairment of DM is the combined result of a repositioning of the reactive carbon of linoleic acid substrate with regard to both the iron cofactor and a catalytically linked dynamic region of protein.
Figures






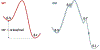
References
-
- Radzicka A; Wolfenden R, A Proficient Enzyme. Science 1995, 267, 90–93. - PubMed
-
- Edwards DR; Lohman DC; Wolfenden R, Catalytic Proficiency: The Extreme Case of S-O Cleaving Sulfatases. J. Am. Chem. Soc 2012, 134, 525–531. - PubMed
-
- Bruice TC, A View at the Millennium: The Efficiency of Enzymatic Catalysis. Acc. Chem. Res 2002, 35, 139–148. - PubMed
-
- Benkovic SJ; Hammes-Schiffer S, A Perspective on Enzyme Catalysis. Science 2003, 301, 1196–1202. - PubMed
-
- Nagel ZD; Klinman JP, A 21st Century Revisionist’s View at a Turning Point in Enzymology. Nat. Chem. Biol 2009, 5, 543–550. - PubMed

