Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus
- PMID: 30645203
- PMCID: PMC6391094
- DOI: 10.1172/JCI124466
Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus
Abstract
Background: Plasmacytoid DCs (pDC) produce large amounts of type I IFN (IFN-I), cytokines convincingly linked to systemic lupus erythematosus (SLE) pathogenesis. BIIB059 is a humanized mAb that binds blood DC antigen 2 (BDCA2), a pDC-specific receptor that inhibits the production of IFN-I and other inflammatory mediators when ligated. A first-in-human study was conducted to assess safety, tolerability, and pharmacokinetic (PK) and pharmacodynamic (PD) effects of single BIIB059 doses in healthy volunteers (HV) and patients with SLE with active cutaneous disease as well as proof of biological activity and preliminary clinical response in the SLE cohort.
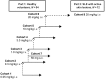
Methods: A randomized, double-blind, placebo-controlled clinical trial was conducted in HV (n = 54) and patients with SLE (n = 12). All subjects were monitored for adverse events. Serum BIIB059 concentrations, BDCA2 levels on pDCs, and IFN-responsive biomarkers in whole blood and skin biopsies were measured. Skin disease activity was determined using the Cutaneous Lupus Erythematosus Disease Area and Severity Index Activity (CLASI-A).
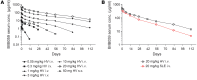
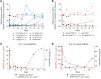
Results: Single doses of BIIB059 were associated with favorable safety and PK profiles. BIIB059 administration led to BDCA2 internalization on pDCs, which correlated with circulating BIIB059 levels. BIIB059 administration in patients with SLE decreased expression of IFN response genes in blood, normalized MxA expression, reduced immune infiltrates in skin lesions, and decreased CLASI-A score.
Conclusions: Single doses of BIIB059 were associated with favorable safety and PK/PD profiles and robust target engagement and biological activity, supporting further development of BIIB059 in SLE. The data suggest that targeting pDCs may be beneficial for patients with SLE, especially those with cutaneous manifestations.
Trial registration: ClinicalTrials.gov NCT02106897.
Funding: Biogen Inc.
Keywords: Dendritic cells; Immunology; Lupus; Pharmacology.
Conflict of interest statement
Figures




Comment in
-
A promising approach to targeting type 1 IFN in systemic lupus erythematosus.J Clin Invest. 2019 Mar 1;129(3):958-961. doi: 10.1172/JCI127101. Epub 2019 Feb 18. J Clin Invest. 2019. PMID: 30776023 Free PMC article.
References
-
- Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30(9):1955–1959. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

