No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study
- PMID: 30645594
- PMCID: PMC6333326
- DOI: 10.1371/journal.pmed.1002725
No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study
Abstract
Background: Studies have shown strong positive associations between serum urate (SU) levels and chronic kidney disease (CKD) risk; however, whether the relation is causal remains uncertain. We evaluate whether genetic data are consistent with a causal impact of SU level on the risk of CKD and estimated glomerular filtration rate (eGFR).
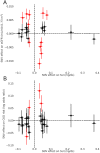
Methods and findings: We used Mendelian randomization (MR) methods to evaluate the presence of a causal effect. We used aggregated genome-wide association data (N = 110,347 for SU, N = 69,374 for gout, N = 133,413 for eGFR, N = 117,165 for CKD), electronic-medical-record-linked UK Biobank data (N = 335,212), and population-based cohorts (N = 13,425), all in individuals of European ancestry, for SU levels and CKD. Our MR analysis showed that SU has a causal effect on neither eGFR level nor CKD risk across all MR analyses (all P > 0.05). These null associations contrasted with our epidemiological association findings from the 4 population-based cohorts (change in eGFR level per 1-mg/dl [59.48 μmol/l] increase in SU: -1.99 ml/min/1.73 m2; 95% CI -2.86 to -1.11; P = 8.08 × 10(-6); odds ratio [OR] for CKD: 1.48; 95% CI 1.32 to 1.65; P = 1.52 × 10(-11)). In contrast, the same MR approaches showed that SU has a causal effect on the risk of gout (OR estimates ranging from 3.41 to 6.04 per 1-mg/dl increase in SU, all P < 10-3), which served as a positive control of our approach. Overall, our MR analysis had >99% power to detect a causal effect of SU level on the risk of CKD of the same magnitude as the observed epidemiological association between SU and CKD. Limitations of this study include the lifelong effect of a genetic perturbation not being the same as an acute perturbation, the inability to study non-European populations, and some sample overlap between the datasets used in the study.
Conclusions: Evidence from our series of causal inference approaches using genetics does not support a causal effect of SU level on eGFR level or CKD risk. Reducing SU levels is unlikely to reduce the risk of CKD development.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: HKC has received funding from grants from Astra-Zeneca during the conduct of the study. HKC has also received funding from grants from Selecta, Horizon Pharma, and Takeda, not related to the submitted work. GN has received funding from grants from Goldfinch Bio, personal fees from pulseData LLC, not related to the submitted work. GN is a co-founder of RenalytixAI and is a member advisory board of RenalytixAI and own equity in the same. TRM has received funding from Ardea Biosciences and Ironwood Pharmaceuticals, not related to the submitted work. RD has received funding from grants from AstraZeneca, during the conduct of the study. RD has also received funding from grants from Goldfinch Bio, not related to the submitted work.
Figures

References
-
- National Kidney Foundation. Global facts: about kidney disease. New York: National Kidney Foundation; 2017. [cited 2018 Dec 13]. https://www.kidney.org/kidneydisease/global-facts-about-kidney-disease.
Publication types
MeSH terms
Substances
Grants and funding
- U01 HG004402/HG/NHGRI NIH HHS/United States
- N01 HC055020/HL/NHLBI NIH HHS/United States
- N01 HC085079/HL/NHLBI NIH HHS/United States
- N01 HC048047/HL/NHLBI NIH HHS/United States
- N01 HC055018/HL/NHLBI NIH HHS/United States
- N01 HC085084/HC/NHLBI NIH HHS/United States
- N01 HC048050/HL/NHLBI NIH HHS/United States
- N01 HC055022/HL/NHLBI NIH HHS/United States
- K23 DK107908/DK/NIDDK NIH HHS/United States
- N01 HC085081/HL/NHLBI NIH HHS/United States
- N01 HC095095/HL/NHLBI NIH HHS/United States
- N01 HC085080/HL/NHLBI NIH HHS/United States
- N01 HC055016/HL/NHLBI NIH HHS/United States
- N01 HC048048/HL/NHLBI NIH HHS/United States
- N01 HC055019/HL/NHLBI NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- U01 HL080295/HL/NHLBI NIH HHS/United States
- N01 HC085082/HL/NHLBI NIH HHS/United States
- R35 GM124836/GM/NIGMS NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- R01 HL059367/HL/NHLBI NIH HHS/United States
- N01 HC055021/HL/NHLBI NIH HHS/United States
- N01 HC085086/HL/NHLBI NIH HHS/United States
- R01 HL139865/HL/NHLBI NIH HHS/United States
- N01 HC085083/HL/NHLBI NIH HHS/United States
- R01 HL086694/HL/NHLBI NIH HHS/United States
- N01 HC048049/HL/NHLBI NIH HHS/United States
- N01 HC085085/HC/NHLBI NIH HHS/United States
- N01 HC055015/HL/NHLBI NIH HHS/United States
- R01 AR065944/AR/NIAMS NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- T32 HL007824/HL/NHLBI NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- R01 AR056291/AR/NIAMS NIH HHS/United States
- P50 AR060772/AR/NIAMS NIH HHS/United States
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- N01 HC075150/HL/NHLBI NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

