The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes
- PMID: 30645667
- PMCID: PMC7237273
- DOI: 10.1007/s00125-018-4800-2
The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes
Erratum in
-
Correction to: The discovery of novel predictive biomarkers and early-stage pathophysiology for the transition from gestational diabetes to type 2 diabetes.Diabetologia. 2019 Apr;62(4):730-731. doi: 10.1007/s00125-019-4827-z. Diabetologia. 2019. PMID: 30734838
Abstract
Aims/hypothesis: Gestational diabetes mellitus (GDM) affects up to 20% of pregnancies, and almost half of the women affected progress to type 2 diabetes later in life, making GDM the most significant risk factor for the development of future type 2 diabetes. An accurate prediction of future type 2 diabetes risk in the early postpartum period after GDM would allow for timely interventions to prevent or delay type 2 diabetes. In addition, new targets for interventions may be revealed by understanding the underlying pathophysiology of the transition from GDM to type 2 diabetes. The aim of this study is to identify both a predictive signature and early-stage pathophysiology of the transition from GDM to type 2 diabetes.
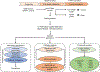
Methods: We used a well-characterised prospective cohort of women with a history of GDM pregnancy, all of whom were enrolled at 6-9 weeks postpartum (baseline), were confirmed not to have diabetes via 2 h 75 g OGTT and tested anually for type 2 diabetes on an ongoing basis (2 years of follow-up). A large-scale targeted lipidomic study was implemented to analyse ~1100 lipid metabolites in baseline plasma samples using a nested pair-matched case-control design, with 55 incident cases matched to 85 non-case control participants. The relationships between the concentrations of baseline plasma lipids and respective follow-up status (either type 2 diabetes or no type 2 diabetes) were employed to discover both a predictive signature and the underlying pathophysiology of the transition from GDM to type 2 diabetes. In addition, the underlying pathophysiology was examined in vivo and in vitro.
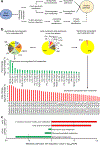
Results: Machine learning optimisation in a decision tree format revealed a seven-lipid metabolite type 2 diabetes predictive signature with a discriminating power (AUC) of 0.92 (87% sensitivity, 93% specificity and 91% accuracy). The signature was highly robust as it includes 45-fold cross-validation under a high confidence threshold (1.0) and binary output, which together minimise the chance of data overfitting and bias selection. Concurrent analysis of differentially expressed lipid metabolite pathways uncovered the upregulation of α-linolenic/linoleic acid metabolism (false discovery rate [FDR] 0.002) and fatty acid biosynthesis (FDR 0.005) and the downregulation of sphingolipid metabolism (FDR 0.009) as being strongly associated with the risk of developing future type 2 diabetes. Focusing specifically on sphingolipids, the downregulation of sphingolipid metabolism using the pharmacological inhibitors fumonisin B1 (FB1) and myriocin in mouse islets and Min6 K8 cells (a pancreatic beta-cell like cell line) significantly impaired glucose-stimulated insulin secretion but had no significant impact on whole-body glucose homeostasis or insulin sensitivity.
Conclusions/interpretation: We reveal a novel predictive signature and associate reduced sphingolipids with the pathophysiology of transition from GDM to type 2 diabetes. Attenuating sphingolipid metabolism in islets impairs glucose-stimulated insulin secretion.
Keywords: Gestational diabetes mellitus; Glucose-stimulated insulin secretion; Lipidomic study; Machine learning; Multiple logistic regression; Pathophysiology; Predictive biomarker; Prospective cohort; Sphingolipid metabolism; Type 2 diabetes.
Figures




References
-
- Gunderson EP, Lewis CE, Tsai AL et al. (2007) A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes 56(12):2990–2996. 10.2337/db07-1024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

