Assessment of Follow-up Care After Emergency Department Presentation for Mild Traumatic Brain Injury and Concussion: Results From the TRACK-TBI Study
- PMID: 30646055
- PMCID: PMC6324305
- DOI: 10.1001/jamanetworkopen.2018.0210
Assessment of Follow-up Care After Emergency Department Presentation for Mild Traumatic Brain Injury and Concussion: Results From the TRACK-TBI Study
Abstract
Importance: Mild traumatic brain injury (mTBI) affects millions of Americans each year. Lack of consistent clinical practice raises concern that many patients with mTBI may not receive adequate follow-up care.
Objective: To characterize the provision of follow-up care to patients with mTBI during the first 3 months after injury.
Design, setting, and participants: This cohort study used data on patients with mTBI enrolled in the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) study between February 26, 2014, and August 25, 2016. We examined site-specific variations in follow-up care, the types of clinicians seen by patients receiving follow-up care, and patient and injury characteristics associated with a higher likelihood of receiving follow-up care. The TRACK-TBI study is a prospective, multicenter, longitudinal observational study of patients with TBI presenting to the emergency department of 1 of 11 level I US trauma centers. Study data included patients with head trauma who underwent a computed tomography (CT) scan within 24 hours of injury, had a Glasgow Coma Scale score of 13 to 15, were aged 17 years or older, and completed follow-up care surveys at 2 weeks and 3 months after injury (N = 831).
Main outcomes and measures: Follow-up care was defined as hospitals providing TBI educational material at discharge, hospitals calling patients to follow up, and patients seeing a physician or other medical practitioner within 3 months after the injury. Unfavorable outcomes were assessed with the Rivermead Post Concussion Symptoms Questionnaire.
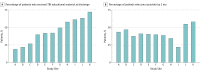
Results: Of 831 patients (289 [35%] female; 483 [58%] non-Hispanic white; mean [SD] age, 40.3 [16.9] years), less than half self-reported receiving TBI educational material at discharge (353 patients [42%]) or seeing a physician or other health care practitioner within 3 months after injury (367 patients [44%]). Follow-up care varied by study site; adjusting for patient characteristics, the provision of educational material varied from 19% to 72% across sites. Of 236 patients with a positive finding on a CT scan, 92 (39%) had not seen a medical practitioner 3 months after the injury. Adjusting for injury severity and demographics, patient admission to the hospital ward or intensive care unit, patient income, and insurance status were not associated with the probability of seeing a medical practitioner. Among the patients with 3 or more moderate to severe postconcussive symptoms, only 145 of 279 (52%) reported having seen a medical practitioner by 3 months.
Conclusions and relevance: There are gaps in follow-up care for patients with mTBI after hospital discharge, even those with a positive finding on CT or who continue to experience postconcussive symptoms.
Conflict of interest statement
Figures
Comment in
-
Mild Traumatic Brain Injury: A Clarion Call for Care of the Postconcussive Spectrum.JAMA Netw Open. 2018 May 18;1(1):e180211. doi: 10.1001/jamanetworkopen.2018.0211. JAMA Netw Open. 2018. PMID: 30646052 No abstract available.
References
-
- Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87.
-
- Coronado VG, Xu L, Basavaraju SV, et al. ; Centers for Disease Control and Prevention (CDC) . Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1-32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

