Age of Data at the Time of Publication of Contemporary Clinical Trials
- PMID: 30646100
- PMCID: PMC6324269
- DOI: 10.1001/jamanetworkopen.2018.1065
Age of Data at the Time of Publication of Contemporary Clinical Trials
Abstract
Importance: As medical knowledge and clinical practice rapidly evolve over time, there is an imperative to publish results of clinical trials in a timely way and reduce unnecessary delays.
Objectives: To characterize the age of clinical trial data at the time of publication in journals with a high impact factor and highlight the time from final data collection to publication.
Design and setting: A cross-sectional analysis was conducted of all randomized clinical trials published from January 1 through December 31, 2015, in the Annals of Internal Medicine, BMJ, JAMA, JAMA Internal Medicine, Lancet, and New England Journal of Medicine. Multivariable linear regression analyses were conducted to assess whether data age (adjusted for follow-up duration) and publication time were associated with trial characteristics.
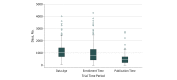
Main outcomes and measures: The outcome measures were the midpoint of data collection until publication (data age), the time from first participant enrollment to last participant enrollment (enrollment time), and the time from final data collection to publication (publication time).
Results: There were 341 clinical trials published in 2015 by the 6 journals. For assessment of the primary end point, 37 trials (10.9%) had a follow-up period of less than 1 month, 172 trials (50.4%) had a follow-up period of 1 month to 1 year, and 132 trials (38.7%) had a follow-up period of more than 1 year. For all trials, the median data age at publication was 33.9 months (interquartile range, 23.5-46.3 months). Among trials with a follow-up period of 1 month or less, the median data age was 30.6 months (interquartile range, 18.6-39.0 months). A total of 68 trials (19.9%) required more than 4 years to complete enrollment. The median time from the completion of data collection to publication was 14.8 months (interquartile range, 7.4-22.2 months); publication time was 2 or more years in 63 trials (18.5%). In multivariable regression analyses adjusted for follow-up time, inconclusive or unfavorable trial results were significantly associated with older data age (>235 days). Compared with trials funded only by private industry, trials funded by government were associated with a significantly longer time to publication (>180 days).
Conclusions and relevance: Clinical trials in journals with a high impact factor were published with a median data age of nearly 3 years. For a substantial proportion of studies, time for enrollment and time from completion of data collection to publication were quite long, indicating marked opportunities for improvement in clinical trials to reduce data age.
Conflict of interest statement
Figures
Similar articles
-
Publication status of contemporary oncology randomised controlled trials worldwide.Eur J Cancer. 2016 Oct;66:17-25. doi: 10.1016/j.ejca.2016.06.010. Epub 2016 Aug 11. Eur J Cancer. 2016. PMID: 27522246
-
Publication Speed, Reporting Metrics, and Citation Impact of Cardiovascular Trials Supported by the National Heart, Lung, and Blood Institute.J Am Heart Assoc. 2015 Jul 31;4(8):e002292. doi: 10.1161/JAHA.115.002292. J Am Heart Assoc. 2015. PMID: 26231845 Free PMC article.
-
Availability of study protocols for randomized trials published in high-impact medical journals: A cross-sectional analysis.Clin Trials. 2020 Feb;17(1):99-105. doi: 10.1177/1740774519868310. Epub 2019 Aug 26. Clin Trials. 2020. PMID: 31450958
-
Global collaborative networks on meta-analyses of randomized trials published in high impact factor medical journals: a social network analysis.BMC Med. 2014 Jan 29;12:15. doi: 10.1186/1741-7015-12-15. BMC Med. 2014. PMID: 24476131 Free PMC article. Review.
-
Publication of noninferiority clinical trials: changes over a 20-year interval.Pharmacotherapy. 2011 Sep;31(9):833-9. doi: 10.1592/phco.31.9.833. Pharmacotherapy. 2011. PMID: 21923583 Review.
Cited by
-
Publication bias in trials registered in the Australian New Zealand Clinical Trials Registry: Is it a problem? A cross-sectional study.PLoS One. 2023 Jan 5;18(1):e0279926. doi: 10.1371/journal.pone.0279926. eCollection 2023. PLoS One. 2023. PMID: 36602999 Free PMC article.
-
Inflection Point: Ideas for Accelerating Breakthroughs and Improving Cardiovascular Health.Circ Cardiovasc Qual Outcomes. 2020 Dec;13(12):e007615. doi: 10.1161/CIRCOUTCOMES.120.007615. Epub 2020 Nov 17. Circ Cardiovasc Qual Outcomes. 2020. PMID: 33200951 Free PMC article. No abstract available.
-
Lessons Learned from National Heart, Lung, and Blood Institute Covid-19 Clinical Trials.NEJM Evid. 2024 Nov;3(11):EVIDctcs2300291. doi: 10.1056/EVIDctcs2300291. Epub 2024 Oct 22. NEJM Evid. 2024. PMID: 39437132 Free PMC article.
-
Guiding Principles for the Conduct of Observational Critical Care Research for Coronavirus Disease 2019 Pandemics and Beyond: The Society of Critical Care Medicine Discovery Viral Infection and Respiratory Illness Universal Study Registry.Crit Care Med. 2020 Nov;48(11):e1038-e1044. doi: 10.1097/CCM.0000000000004572. Crit Care Med. 2020. PMID: 32932348 Free PMC article.
-
Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals.Korean J Radiol. 2018 Nov-Dec;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172. Epub 2018 Oct 18. Korean J Radiol. 2018. PMID: 30386148 Free PMC article.
References
-
- Mina A, Ramlogan R, Tampubolon G, Metcalfe JS. Mapping evolutionary trajectories: applications to the growth and transformation of medical knowledge. Res Policy. 2007;36(5):-. doi:10.1016/j.respol.2006.12.007 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

