Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysis
- PMID: 30646241
- PMCID: PMC6324456
- DOI: 10.1001/jamanetworkopen.2018.3485
Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysis
Abstract
Importance: Cannabinoids have antispastic and analgesic effects; however, their role in the treatment of multiple sclerosis (MS) symptoms is not well defined.
Objective: To conduct a systematic review and meta-analysis to assess the efficacy and tolerability of medicinal cannabinoids compared with placebo in the symptomatic treatment of patients with MS.
Data sources: MEDLINE and the Cochrane Library Plus up to July 26, 2016. No restrictions were applied. The search was completed with information from ClinicalTrials.gov.
Study selection: Randomized, double-blind, and placebo-controlled trials evaluating the effect of medicinal cannabinoids by oral or oromucosal route of administration on the symptoms of spasticity, pain, or bladder dysfunction in adult patients with MS.
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines were followed. Effect sizes were calculated as standardized mean difference (SMD) for efficacy, and rate ratio (RR) for tolerability. Within each study, those SMDs evaluating the same outcome were combined before the meta-analysis to obtain a single value per outcome and study. Pooling of the studies was performed on an intention-to-treat basis by means of random-effect meta-analysis.
Main outcomes and measures: Spasticity (on the Ashworth and Modified Ashworth scales and subjective), pain, bladder dysfunction, adverse events, and withdrawals due to adverse events.
Results: Seventeen selected trials including 3161 patients were analyzed. Significant findings for the efficacy of cannabinoids vs placebo were SMD = -0.25 SD (95% CI, -0.38 to -0.13 SD) for spasticity (subjective patient assessment data), -0.17 SD (95% CI, -0.31 to -0.03 SD) for pain, and -0.11 SD (95% CI, -0.22 to -0.0008 SD) for bladder dysfunction. Results favored cannabinoids. Findings for tolerability were RR = 1.72 patient-years (95% CI, 1.46-2.02 patient-years) in the total adverse events analysis and 2.95 patient-years (95% CI, 2.14-4.07 patient-years) in withdrawals due to adverse events. Results described a higher risk for cannabinoids. The serious adverse events meta-analysis showed no statistical significance.
Conclusions and relevance: The results suggest a limited efficacy of cannabinoids for the treatment of spasticity, pain, and bladder dysfunction in patients with MS. Therapy using these drugs can be considered as safe.
Trial registration: PROSPERO Identifier: CRD42014015391.
Conflict of interest statement
Figures

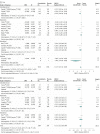
Comment in
-
Cannabinoids for Symptoms of Multiple Sclerosis: Benefits to Patients Still Unclear.JAMA Netw Open. 2018 Oct 5;1(6):e183484. doi: 10.1001/jamanetworkopen.2018.3484. JAMA Netw Open. 2018. PMID: 30646233 No abstract available.
References
-
- Multiple Sclerosis International Federation Atlas of MS 2013. London, United Kingdom: Summers Editorial & Design; 2013.
-
- GW Pharmaceuticals plc. Sativex® (delta-9-tetrahydrocannibinol and cannabidiol in the EU) (nabiximols in the USA). https://www.gwpharm.com/healthcare-professionals/sativex®-delta-9-tetrah.... Accessed August 10, 2018.
-
- GW Pharmaceuticals plc. Health Canada grants full approval of Sativex for the treatment of spasticity due to multiple sclerosis. https://www.gwpharm.com/about-us/news/health-canada-grants-full-approval.... Accessed August 10, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

