Assessment of Validity of a Blood-Based 3-Gene Signature Score for Progression and Diagnosis of Tuberculosis, Disease Severity, and Treatment Response
- PMID: 30646264
- PMCID: PMC6324428
- DOI: 10.1001/jamanetworkopen.2018.3779
Assessment of Validity of a Blood-Based 3-Gene Signature Score for Progression and Diagnosis of Tuberculosis, Disease Severity, and Treatment Response
Abstract
Importance: The World Health Organization identified the need for a non-sputum-based triage test to identify those in need of further tuberculosis (TB) testing.
Objective: To determine whether the 3-gene TB score can be a diagnostic tool throughout the course of TB disease, from latency to diagnosis to treatment response, and posttreatment residual inflammation.
Design, setting, and participants: This nested case-control study analyzed the 3-gene TB score in 3 cohorts, each focusing on a different stage of TB disease: (1) the Adolescent Cohort Study profiled whole-blood samples from adolescents with latent Mycobacterium tuberculosis infection, some of which progressed to active TB (ATB), using RNA sequencing; (2) the Brazil Active Screen Study collected whole blood from an actively screened case-control cohort of adult inmates from 2 prisons in Mato Grosso do Sul, Brazil, for ATB from January 2016 to February 2016; and (3) the Catalysis Treatment Response Cohort (CTRC) identified culture-positive adults in primary health care clinics in Cape Town, South Africa, from 2005 to 2007 and collected whole blood for RNA sequencing from patients with ATB at diagnosis and weeks 1, 4, and 24. The CTRC patients also had positron emission tomography-computed tomography scans at diagnosis, week 4, and week 24. Analyses were performed from September 2017 to June 2018.
Main outcomes and measures: A 3-gene messenger RNA expression score, measured by quantitative polymerase chain reaction or RNA sequencing, was evaluated for distinguishing the following: individuals who progressed to ATB from those who did not, individuals with ATB from those without, and individuals with slower treatment response during TB therapy.
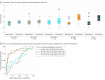
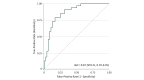
Results: Patients evaluated in this study included 144 adolescents from the Adolescent Cohort Study (aged 12-18 years; 96 female and 48 male), 81 adult prison inmates from the Brazil Active Screen Study (aged 20-72 years; 81 male), and 138 adult community members from the CTRC (aged 17-64 years; 81 female and 57 male). The 3-gene TB score identified progression from latent M tuberculosis infection to ATB 6 months prior to sputum conversion with 86% sensitivity and 84% specificity (area under the curve [AUC], 0.86; 95% CI, 0.77-0.96) and patients with ATB in the Brazil Active Screen Study cohort (AUC, 0.87; 95% CI, 0.78-0.95) and CTRC (AUC, 0.94; 95% CI, 0.88-0.99). It also identified CTRC patients with failed treatment at the end of treatment (AUC, 0.93; 95% CI, 0.83-1.00). Collectively, across all cohorts, the 3-gene TB score identified patients with ATB with 90% sensitivity, 70% specificity, and 99.3% negative predictive value at 4% prevalence.
Conclusions and relevance: Across 3 independent prospective cohorts, the 3-gene TB score approaches the World Health Organization target product profile benchmarks for non-sputum-based triage test with high negative predictive value. This gene expression diagnostic approach should be considered for further validation and future implementation.
Conflict of interest statement
Figures


References
-
- World Health Organization High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting. Geneva, Switzerland: World Health Organization; 2014.
-
- World Health Organization Consensus Meeting Report: Development of a Target Product Profile (TPP) and a Framework for Evaluation for a Test for Predicting Progression From Tuberculosis Infection to Active Disease. Geneva, Switzerland: World Health Organization; 2017.
-
- Seshadri P, Denkinger C.. Draft target product profile: test for progression of tuberculosis infection. http://www.finddx.org/wp-content/uploads/2016/05/TPP-LTBIprogression.pdf. Accessed September 10, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

