Assessment of Pharmaceutical Company and Device Manufacturer Payments to Gastroenterologists and Their Participation in Clinical Practice Guideline Panels
- PMID: 30646328
- PMCID: PMC6324539
- DOI: 10.1001/jamanetworkopen.2018.6343
Assessment of Pharmaceutical Company and Device Manufacturer Payments to Gastroenterologists and Their Participation in Clinical Practice Guideline Panels
Abstract
Importance: Payments from pharmaceutical and device manufacturers to physicians may influence the advice physicians give patients and peers.
Objectives: To investigate the nature and amounts of monetary and other benefits that gastroenterologists received and to determine the participation of those receiving benefits in the formulation of clinical practice guidelines.
Design, setting, and participants: This cohort study analyzed information from the Centers for Medicare & Medicaid Services Open Payments database, including all reports about payments that pharmaceutical and device manufacturers gave to adult or pediatric gastroenterologists in 2016. PubMed was used to examine the professional affiliations and publication records of top payment recipients. Panelists of clinical guidelines who also received personal financial rewards listed in the Open Payments database were identified.
Main outcomes and measures: Payments made to gastroenterologists by pharmaceutical company and device manufacturers.
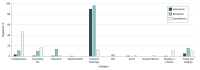
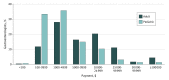
Results: Of 15 497 gastroenterologists, 13 467 (86.9%) received a total of 432 463 payments accounting for a total expenditure of $67 144 862. Direct financial payments for consultations, talks, or other services were made to 2055 physicians and were responsible for 4.2% of payments (18 179 of 432 463), but for 62.7% of total expenditures ($42 086 207 of $67 144 862). Although a significant number of submissions were for food and beverages, they constituted only a small amount of total expenditure. For gastroenterologists treating adult patients, 10 products were linked to 63.8% of payments (11 221 of 17 588) related to direct financial rewards and 37.1% of the total expenditures ($24 892 643 of $67 144 862). Twenty-nine of 36 clinical practice guidelines included panelists who had received honoraria or consultation fees from industry sources, with amounts exceeding $10 000 in 8 of them (22%).
Conclusions and relevance: Most gastroenterologists accept meals or gifts from industry, with 2055 of 15 497 gastroenterologists receiving direct payments and 8 of 36 clinical practice guidelines panelists having received more than $10 000. Considering the known impact of such benefits on prescribing patterns and other professional behaviors, policy makers should consider revising regulations governing interactions with industry and disclosure formats alerting others to their potential biasing impact.
Figures

Comment in
-
The Call for Greater Transparency in Conflicts of Interest.JAMA Netw Open. 2018 Dec 7;1(8):e186342. doi: 10.1001/jamanetworkopen.2018.6342. JAMA Netw Open. 2018. PMID: 30646322 No abstract available.

